E S P
440 likes | 697 Vues
E S P. E lectronic medical record S upport for P ublic health. Integrated Surveillance Seminar Series National Center for Public Health Informatics December 12, 2007. Michael Klompas MD, MPH, FRCPC CDC Center of Excellence in Public Health Informatics (NCPHI PH000238D)

E S P
E N D
Presentation Transcript
E S P Electronic medical recordSupport forPublic health Integrated Surveillance Seminar SeriesNational Center for Public Health Informatics December 12, 2007 Michael Klompas MD, MPH, FRCPC CDC Center of Excellence in Public Health Informatics (NCPHI PH000238D) Harvard Medical School, Boston, MA
CDC Center of Excellence in Public Health Informatics (Boston)funded by the National Center for Public Health Informatics • Harvard Medical School / Harvard Pilgrim Health Care Department of Ambulatory Care and Prevention • Children’s Hospital Informatics Program • Massachusetts Department of Public Health • Harvard Vanguard Medical Associates (for Atrius Health) • Brigham and Women’s Hospital Channing Laboratory
“No health department, State or local, can effectively prevent or control disease without knowledge of when, where, and under what conditions cases are occurring” Introductory statement printed each week inPublic Health Reports, 1913-1951
The evolution of notifiable disease reporting • Traditional paper based reporting • Clinically detailed • Slow, often incomplete, labour intensive, dependent on clinician initiative • Web based notifiable disease reporting • Great improvement in speed and accessibility of data (received in electronic form) • But still requires clinician initiative to report • Electronic laboratory based reporting • Fast, accurate, often digital, no need for clinician initiative
Limitations of Electronic Laboratory Reporting • Often missing detailed demographic information on patient and clinician contact details • No information on patient symptoms, pregnancy status, or prescribed treatment • Typically does not integrate multiple tests to yield a diagnosis • e.g. negative HIV ELISA and high viral load = acute HIV • No clues that lab test might be false positive • e.g. positive Hep A IgM but no order for liver function tests • Cannot report purely clinical diagnoses • e.g. Pelvic inflammatory disease, Lyme erythema migrans • Typically generates multiple reports on the same patient for the same condition • e.g. chronic hepatitis B
Our goal • Combine the best of traditional clinician-initiated reporting and electronic laboratory reporting systems: • Fast, accurate, clinically detailed, digital reports Clinician initiated manual reporting Electronic laboratory reporting Automated disease detection and reporting from electronic medical records
Allied goals • Create a generalizable architecture for disease detection and reporting that is agnostic to the source EMR system • Digitize notifiable disease reporting at the provider level to potentially feed NEDSS reporting from states to CDC
Electronic Support for Public health (ESP) • Software and architecture to automate detection and reporting of notifiable diseases • Surveys codified electronic medical record data for patients with notifiable conditions • Generates and sends secure case HL7 reports to the health department
Health Department diagnoses lab results HL7 electronic case reports of notifiableconditions meds vital signs D P H demographics ESP: Automated detection and reporting of notifiable conditions Practice EMR’s ESP Server
ESP Decoupled architecture ESP decoupled from host electronic medical record (EMR) EMR
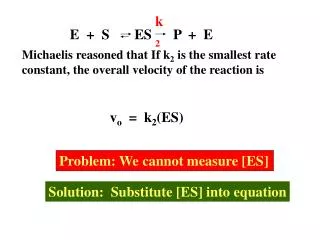
Case Management Interface • All potential cases available for review by infection control personnel prior to transmission to the health department (optional functionality)
Report to Health Department • Patient demographics • Responsible clinician, site, contact info • Basis for condition being detected • Treatment given • Symptoms (ICD9 code & temperature) • Pregnancy status (if pertinent)
Atrius Health 27 multispecialty practices in MA EPIC EMR ~600,000 patients >500 clinicians ESP server resides in the central data processing center Analyzes data from all 27 sites Current Status: Operational in Atrius HealthJanuary 2007 to present Boston, MA © Google Maps
Current Status • Currently reporting chlamydia, gonorrhea, pelvic inflammatory disease, and acute hepatitis A. To date: • 1143 cases of chlamydia • 151 cases of gonorrhea • 25 cases of pelvic inflammatory disease • 6 cases of acute hepatitis A • Definitions under validation for: • Acute and chronic hepatitis B • Acute hepatitis C • Tuberculosis
Case Identification • Logical combinations of laboratory test results, diagnostic codes, vital signs, and / or medication prescriptions • Case definitions tested and refined against up to 18 years of historical EMR data • Charts reviewed on all patients identified by algorithms • Comparison with Massachusetts DPH disease lists to identify patients missed by the algorithms • Repeatedly refine algorithm to maximize accuracy
Case Identification Logic:Chlamydia Positive test for any of the following:
Case Identification Logic:Acute Hepatitis B • Both of the following: • ICD9 for jaundice OR liver function tests > 5x normal • IgM to core antigen OR • All five of the following: • ICD9 for jaundice OR liver function tests > 5x normal • Bilirubin ≥1.5 • Hep B surface antigen or ‘e’ antigen present • No prior positive Hep B specific lab tests • Absence of ICD9 code for chronic hepatitis B OR • Transition from negative to positive Heb B surface antigen
Case Identification LogicActive Tuberculosis • Any of the following: • Prescription for pyrazinamide OR • Order for AFB smear or culture followed by ICD9 code for TB within 60 days OR • Order for 2 or more anti-tuberculous medications followed by an ICD9 code for TB within 60 days
Manual versus electronic reportingAtrius Health, June 2006 - July 2007 *generated by dedicated infection control reporting staff
Manual versus electronic reportingAtrius Health, June 2006 - July 2007
Manual versus electronic reportingAtrius Health, June 2006 - July 2007 * Including transposition of first and last name, incorrect first name, and spelling errors * EMR spelling presumed as gold standard
Clinical details on false positive cases • Pelvic inflammatory disease • Pelvic pain, positive cultures for Herpes simplex and Chlamydia • Acute Hepatitis A • Young woman with 10 days pharyngitis and fatigue, monospot negative, HAV IgM+ and EBV VCA IgM+ • Tuberculosis • Patient exposed to MDR TB but no active disease • Patient with prior history of TB presenting with hemoptysis and nodules on chest radiograph
5 acute 133chronic cases 138 distinct patients 600 positive test results for hepatitis B Sorting through positive Hep B Results - ESP versus ELR E S P E L R
Missed Cases • 5 cases known to DPH missed by ESP (versus 266 cases known to ESP but missed by DPH) • 0.6% of all known cases • All missed cases were tests that were edited after placement into EMR – updated results were not forwarded to ESP • 11 cases missed during upgrade of source EMR due to transient interruption of data flow to ESP • Subsequently discovered and retrieved
Next Stepsadd more conditions • Additional diseases to be added to ESP • In progress: • Lyme disease • Measles • Mumps • Rubella • and others…
Protocol for vaccine preventable diseases • Measles / mumps / rubella • Report any patient with ICD9 code or lab order for IgM to measles / mumps / rubella • ICD9 code and lab orders are proxies for clinician suspicion • Immediate reporting to jump start public health investigation • Include patient’s immunization history in the report • Include clinician contact number to facilitate investigation • Simultaneously send ordering clinician a brief electronic questionnaire on patient exposures, symptoms, etc. that ESP will immediately forward to public health
Next StepsNew applications to broaden utility of the ESP platform • Vaccine adverse event surveillance and reporting • Prospective surveillance of patients given a vaccine for 30 days • Seek novel diagnoses, suggestive biochemical changes, and new vaccine allergies suggestive of possible vaccine adverse effect • Elicit clinician comment on purported adverse reaction • Immediate electronic reporting to VAERS if clinician agrees
Next StepsNew applications to consider • The ESP model could also be a suitable platform for other public health priorities • Patient safety initiatives • e.g. follow-up on critical test results, drug interactions, renal dose adjustments, medication adverse effects, missing health maintenance activities, vaccine registries… • Syndromic surveillance • Asthma surveillance and cluster detection • Add insurance claims to increase the robustness and completeness of disease identification
Next stepsimplement ESP in a new site • Planning underway to implement ESP in the health information exchange of North Adams, MA (serving 14 local practices) • Different EMR, different user culture North Adams Boston © Google Maps
Next StepsDisseminating ESP beyond Massachusetts • ESP software is freely available under a lesser general public license But… • Installation and maintenance of new ESP systems will require significant IT, epidemiologic, and administrative expertise and resources • Is this a role for CDC?
Barriers to broader implementation of ESP • Only about 35% of multi-physician practices have EMR’s • Limited breadth of information capture by many EMR’s • Different coding nomenclature & cultures in different EMR’s • Constant influx of new lab, diagnosis, and med codes • Absence of standardized disease definitions tailored to electronic data • Absence of standardized reporting elements for most diseases • Paucity of resources to support implementation and support of ESP-like systems • Public wariness of electronic surveillance and health reporting
Heterogenous EMR systems • Problem: • Vast array of different EMR systems on the market with different capabilities and operating protocols • Solution: • ESP decoupled from the host EMR to permit compatibility with multiple different EMR systems • Host EMR need only be capable of exporting plain text files with recent encounter data
Heterogenous coding practices • Problem: • Different EMR systems use different coding systems • Coding often arbitrary and idiosyncratic • Solution: • Map proprietary codes to universal nomenclatures • LOINC, SNOMED, ICD9, NDC • Only need to map codes pertinent to notifiable disease detection • thus far about 30 code maps in ESP
Local codes mapped to universal codesCPT to LOINC mapping (Atrius Health)
CPT to LOINC Map - Challenges Proprietary code Obsolete code Multiple codes for same test Incorrect code
New lab and drug codes • Problem: • New lab and drug codes constantly being added to EMR’s • Solution: • ESP constantly scans all incoming data to identify new candidate codes -----Original Message----- From: espuser@lkenpesp.healthone.org Sent: September 27, 2007 8:18 AM To: Klompas, Michael,M.D. Subject: ESP management on 2007-09-27 12:17:39.187975 New (CPT,COMPT,ComponentName): [('87591', '4323', 'NEISSERIA GONORRHOEAE, DNA, SDA, OTV')]
Standardization and Maintenanceof Disease Definitions • Problem: • Currently no standardized definitions for identification of notifiable diseases from EMR data • Standardization essential for data comparability across sites • Validation of definitions requires large populations to assure algorithm accuracy for rare diseases • Possible solutions: • A role for CDC? CSTE? Health departments? Academics? • CDC and CSTE already collaborating to define electronic reporting elements for notifiable diseases
Dissemination of ESP-like systems • Problem: • Where should disease detection and reporting be integrated into the health care system? • Possible solutions: • Integrate ESP logic into EMR software • Make notifiable disease reporting a HITSP standard for EMR certification • Install ESP-like systems in regional health information exchanges • Can CDC lead and support this effort? • Use ESP case identification definitions on Biosense data
ESP Team • Harvard Medical School / Harvard Pilgrim Health Care Department of Ambulatory Care and Prevention • Richard Platt MD, MSc Ross Lazarus MBBS, MPH, MMed • Julie Dunn MPH Michael Calderwood MD • Ken Kleinman ScD Yury Vilk PhD • Kimberly Lane MPH • Harvard Vanguard Medical Associates • Francis X. Campion MD • Benjamin Kruskal MD, PhD • Massachusetts Department of Public Health • Alfred DeMaria MD Bill Dumas RN • Gillian Haney MPH Daniel Church MPH • James Daniel MPH Dawn Heisey MPH • Channing Laboratory of Brigham and Women’s Hospital • Xuanlin Hou MSc Collaborators Wanted! Contact: mklompas@partners.org