Balanced Chemical Equations
170 likes | 328 Vues
Balanced Chemical Equations. Your Turn #1: What happened in Lakehurst, NJ on May 6 th , 1937? (click here to find out; requires Internet connection). Answer:

Balanced Chemical Equations
E N D
Presentation Transcript
Balanced Chemical Equations Your Turn #1: What happened in Lakehurst, NJ on May 6th, 1937? (click here to find out; requires Internet connection) Answer: The German airship Hindenburg exploded while landing at the naval air station in Lakehurst. On board were 61 crew and 36 passengers. 35 people died in the crash. There is still controversy as to what caused the explosion. More recent arguments contend that it was the coating on the surface of the ship that ignited. Others believe it was the gas which kept the Hindenburg afloat that exploded. To see an additional 5-6 minute video of Hindenburg footage click here. Your Turn #2: Even if the gas was not the cause of the initial fire, it would have eventually ignited. What was the name of this highly flammable gas? (click for answer) Answer: hydrogen (today's blimps use the much safer helium)
Balanced Chemical Equations Your Turn #4: When something burns or explodes, what does it generally react with (name and formula)? (click for answer) Your Turn #5: What would you predict is the product when hydrogen reacts with oxygen? (click for answer) Your Turn #3: What is the formula of hydrogen gas? (click for answer) Your Turn #7: What is the general term given to all substances that appear to the right of the arrow? (click for answer) Your Turn #6: What is the general term given to all substances that appear to the left of the arrow? (click for answer) reactants products H2 + O2 H2O oxygen The plus sign means “reacts with”. The arrow means “to produce”.
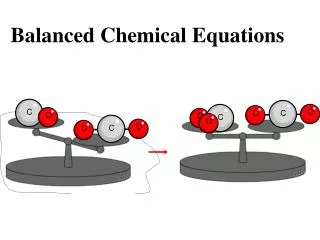
Balanced Chemical Equations To be balanced, a chemical equation has to have the same number of atoms of each element on the left as on the right. This is not the case here because there is one more oxygen on the left than the right. Your Turn #8: How many atoms of hydrogen and how many atoms of oxygen appear on the reactants side of the equation? (click for answer) Your Turn #9: How many atoms of hydrogen and how many atoms of oxygen appear on the products side of the equation? (click for answer) What you see is an example of a chemical equation. However, it is not a balanced chemical equation. 2 hydrogen2 oxygen 2 hydrogen1 oxygen H2 + O2 H2O
Balanced Chemical Equations So the question is, how can we balance an equation? First we will show you the WRONG thing to do. 2 hydrogen2 oxygen 2 hydrogen1 oxygen H2 + O2 H2O
Balanced Chemical Equations So the question is, how can we balance an equation? First we will show you the WRONG thing to do. 2 hydrogen2 oxygen 2 hydrogen2oxygen H2 + O2 H2O2 Wrong!
Balanced Chemical Equations So the question is, how can we balance an equation? First we will show you the WRONG thing to do. 2 hydrogen2 oxygen 2 hydrogen2oxygen H2 + O2 H2O2 I said NEVER! Were you listening???...The reason you can’t change the subscripts is because you are then changing what substance is represented. H2O2 is hydrogen peroxide and a completely different substance then what the product really was (H2O, water). Click to go back to what we had before. NEVER change the subscripts when trying to balance a chemical equation.
Balanced Chemical Equations We balance an equation by adding coefficients (numbers which appear in front of a formula). For example let’s add a 2 in front of H2O (click to add). 2 hydrogen2 oxygen 2 hydrogen1 oxygen H2 + O2 H2O
Balanced Chemical Equations Notice that this does make two oxygen on the right (you multiply the coefficient, in this case 2, by the subscript for oxygen, in this case 1). Now oxygen is balanced! But…. 2 hydrogen2 oxygen 4 hydrogen2 oxygen H2 + O2 H2O 2
Balanced Chemical Equations …it also messes up the hydrogen!! Now there are 4 hydrogen on the right (coefficient of 2 multiplied by a subscript of 2) and 2 hydrogen on the left. So we need to fix the hydrogen… 2 hydrogen2 oxygen 4 hydrogen2 oxygen H2 + O2 H2O 2
Balanced Chemical Equations Your Turn #10: Predict how we can now balance the hydrogen. (click for answer) 2 hydrogen2 oxygen 4 hydrogen2 oxygen H2 + O2 H2O 2
Balanced Chemical Equations Your Turn #10: Predict how we can now balance the hydrogen. (click for answer) 4 hydrogen2 oxygen 4 hydrogen2 oxygen H2 + O2 H2O 2 2 By adding the 2 in front of H2, we now make 4 hydrogen on the left hand side of the equation. This balances the 4 hydrogen on the right. Since the oxygen are also balanced, we now have a balanced equation!
Balanced Chemical Equations Remember, we balance equations by adding coefficients, NOT by changing the subscripts. Below, the coefficient on O2 is assumed to be 1. 4 hydrogen2 oxygen 4 hydrogen2 oxygen H2 + O2 H2O 2 2 coefficients
Balanced Chemical Equations Before you go on to practice balancing more equations, it is worth noting that there really is no advantage to starting on the left or right hand side of the equation. It pretty much is just a trial and error process until you get it balanced. Having said that, there are a few hints that will help you generally and we’ll cover two of them in this lesson. The first is, in general, it is best to balance the more complicated formula(s) first (for example, you would generally balance C6H12O6 before you balance O2). The second hint relates to polyatomic ions and we’ll get to that after you practice a few others first. Your Turn #11: Balance the following equations. (click for answer one equation at a time) Cl2 + H2 HCl 2 Mg + AgCl MgCl2 + Ag (hint: start with AgCl or MgCl2 because they are more complicated than Mg or Ag) 2 2 2 BiCl3 + H2S Bi2S3 + HCl (hint: HCl looks the least complicated so start with one of the others) 3 6
Balanced Chemical Equations Your Turn #12: Balance the following equations. (click for answer one equation at a time) MgCO3 CO2 + MgO already balanced! C3H8 + O2 CO2 + H2O (hint: which one of these formulas do you think is least complicated, thus suggesting you’ll want to balance that last, or at least not first) 5 3 4 2 Al + HCl AlCl3 + H2 6 2 3 Now for your second hint. It relates to equations which contain polyatomic ions. In particular for equations which have the same polyatomic ion on the left as the right. In these cases, it is easiest to balance the polyatomic ion as a complete unit, not element by element. To see what I mean, try Assisted Problem #1 on the next slide.
Balanced Chemical Equations Assisted Problem #1: Balance the following equation. (click for answer one step at a time) Al2(SO4)3 + KOH Al(OH)3 + K2SO4 6 2 3 6 hydroxide (OH-)6 potassium 6 potassium3 sulfate (SO42-) 2 aluminum6 hydroxide (OH-) This makes 6 hydroxide on the left to balance the 6 on the right. However, it also makes 6 K. Predict how we can adjust the right side of the equation to balance the K (click for answer). Now there are 6 K on both sides of the equation (because the coefficient of 3 times the subscript of 2 equals 6). But this also makes 3 sulfates (SO42-). This is good though because there are already three sulfates on the left hand side of the equation! This equation is now balanced! By adding 2 as a coefficient, this makes 2 Al on the right to go with the 2 on the left. But now think of the oxygen and hydrogen in Al(OH)3 not as individual elements but as part of a hydroxide unit. This would make 6 hydroxides (OH-). 6 because the coefficient of 2 is multiplied by the subscript of 3 for the hydroxide. Now predict how we can adjust the left side of the equation in order to balance the hydroxides. Remember, you are trying to balance the OH- as a complete group. For now, don’t think of it as O and H separate from one another (click for answer).
Balanced Chemical Equations Your Turn #13: Balance the following equations (remember to balance the polyatomic ions as complete units). (click for answer one equation at a time) 3 Ca + H3PO4 Ca3(PO4)2 + H2 2 3 6 H 2 PO43- 3 Ca 2 PO43- think of as 3 Ca 6 H 2 NH4Cl + Pb(NO3)2 NH4NO3 + PbCl2 2 1 Pb 2 NO3- 2 NH4+ 2 NO3- think of as 2 NH4+ 2 Cl- 1 Pb 2 Cl-
Balanced Chemical Equations Review Questions: Go to this link to practice balancing equations. Start with #’s 2 and 20 (remember to save the least complicated formula(s) for last) and #’s 7 and 17 (remember to balance polyatomic ions as complete units). You can check your answers by clicking the “Check” button on the bottom of the page. The End