20.7 Naming Alkenes & Alkynes
210 likes | 1.1k Vues
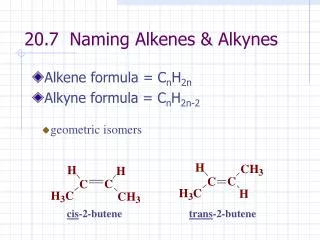
geometric isomers. cis -2-butene. trans -2-butene. 20.7 Naming Alkenes & Alkynes. Alkene formula = C n H 2n Alkyne formula = C n H 2n-2. Select the longest continuous chain of C atoms that contains the double or triple bond.

20.7 Naming Alkenes & Alkynes
E N D
Presentation Transcript
geometric isomers cis-2-butene trans-2-butene 20.7 Naming Alkenes & Alkynes • Alkene formula = CnH2n • Alkyne formula = CnH2n-2
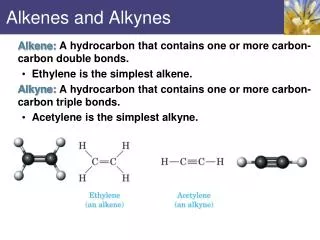
Select the longest continuous chain of C atoms that contains the double or triple bond. • For an alkene, the root name of the carbon chain is the same as for the alkane, except that the –ane is replaced by –yne. For example, for a 2-C chain we have: CH3CH3 CH2=CH2 CH CH Ethane Ethene Ethyne
Number the parent chain, starting at the end closest to the double or triple bond. The location of the multiple bond is given by the lowest-numbered carbon involved in the bond. CH2=CHCH2CH3 CH3CH=CHCH3 1 2 3 4 1 2 3 4 1-butene 2-butene
cyclobutane • Substituents on the parent chain are treated the same way as in naming alkanes. For example, the molecule ClCH=CHCH2CH3 is called 1-chloro-1-butene. • When the carbons are in a circle use the prefix cyclo-
Reactions • Key characteristic of alkenes & alkynes – very reactive around double or triple bond. • Hydrogenation – Use H2 to add H to each C formerly in a double bond
Reactions • Halogenation – involves addition of halogen (group 7) to double bond. CH2=CHCH3 + Br2 CH2BrCHBrCH3 • Polymerization – add together many small molecules (ethene) to get a long chain.