Radiogenic Isotope Geochemistry
150 likes | 637 Vues
Radiogenic Isotope Geochemistry. Lecture 29 Introduction & Physics of the Nucleus. Radiogenic Isotope Geochemistry.

Radiogenic Isotope Geochemistry
E N D
Presentation Transcript
Radiogenic Isotope Geochemistry Lecture 29 Introduction & Physics of the Nucleus
Radiogenic Isotope Geochemistry • Radiogenic isotope geochemistry has its origins in the late 19th century with the discovery of radioactive decay and in the early 20th century with the discovery of the nuclear structure of the atom. • Its initial success was demonstrating the antiquity of the Earth - much older than physicists had thought (geologists won this one) because: • It provided a source of energy to explain heat flowing out of the Earth and • It provide a direct means of dating rocks, and hence geologic events, and putting numbers on the relative time scale geologists had worked out in the 19th century. • Before we begin, we need to review a bit of nuclear physics, which is important not only in understanding radiogenic isotope geochemistry, but also the origin of the elements and their abundances.
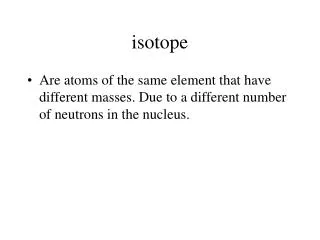
Some terms • Z: Proton number (= atomic number) • N: Neutron number • A: mass number (= N+Z) • M: atomic mass (not an integer, like the above). • Isotopes: • nuclei with the same Z but different N • Isobars: • nuclei with the same A, but different Z, N • Isotone: • nuclei with same N, but different Z • (not used much)
Nuclear Forces • Not all possible combinations of protons and neutrons result in stable nuclei. • Typically for stable nuclei, N ≈ Z. • Thus a significant portion of the nucleus consists of protons, which tend to repel each other. Another force must exist that is stronger than coulomb repulsion at short distances. It must be negligible at larger distances, otherwise all matter would collapse into a single nucleus. • This force, called the nuclear force, is a manifestation of one of the fundamental forces of nature, called the strong force. • If this force is assigned a strength of 1, then the strengths of other forces are: electromagnetic 10-2; weak force 10-5; gravity 10-39. • The nuclear force is mediated by the pion.
Nuclear Binding Energy • A general physical principle is that the lowest energy configuration is the most stable. We would thus expect that if 4He is stable relative to 2 free neutrons and 2free protons, 4He must be a lower energy state compared with the free particles. If this is the case, then from Einstein’s mass–energy equivalence: • E = mc2 • we predict that the 4He nucleus will have less mass than 2 free neutrons and 2 free protons. It does in fact have less mass. • From the principle that the lowest energy configurations are the most stable and the mass–energy equivalence, we should be able to predict the relative stability of various nuclei from their masses alone. • The mass decrement of an atom is δ= W -M • where W is the sum of the mass of the constituent particles and M is the actual mass. • For4He for example, the δ= 0.030306 u. Converting this to energy yields 28.29 MeV. This energy is known as the binding energy. • Dividing by A, the mass number, or number of nucleons, gives the binding energy per nucleon, Eb: The mass decrement for 4He is about 1%, (10-2). The mass decrement associated with binding an electron to a nucleus is of the order of 10-8, so bonds between nucleons are about 106 times stronger than bonds between electrons and nuclei.
Nuclear Stability: The Liquid Drop Model • Why are some nuclei more stable than others? The answer has to do with the forces between nucleons and how nucleons are organized within the nucleus. • The simplest model nucleus is Bohr’s liquid-drop model. It assumes all nucleons have equivalent statesand treats the binding between nucleons as similar to the binding between molecules in a liquid drop, in which there are three effects: • a ‘volume’ energy: energy needed to unbind or evaporate the nucleus: proportional to # of nucleons. • a surface energy: a nucleon in the interior of the nucleus is surrounded by other nucleons and exerts no force on more distance nucleons. But at the surface, the force is unsaturated, leading to a force similar to surface tension. This force tends to minimize the surface area of the nucleus and is strongest for light nuclei and becomes rapidly less important for heavier nuclei. • a coulomb energy: repulsive force between protons. It is proportional to the total number of proton pairs (Z(Z -1)/2) and inversely proportional to radius
Shell Model • Two observations suggest nucleons do not have equivalent states and that instead the nucleus has some kind of structure: • Nuclei with even number of protons and/or neutrons are more stable than those with odd numbers. • Nuclei with magic numbers of nucleons 2, 8, 20, 28, 50, 82, and 126) are more stable and stable nuclei with such magic numbers are particularly common. • These observations lead to the shell model of the nucleus which is much like the shell model of the atom. • Two nucleons can be accepted into each ‘orbit’ and the nucleus is more stable when this happens and the spins cancel. • Nuclei, like atoms, are particularly stable when a ‘shell’ (consisting of a variety of orbits) is filled.
Decay of excited and unstable nuclei • If we randomly throw protons and neutrons together to try to form a nucleus, there are three possible outcomes: • No nucleus can form from that particular combination of Z and N • A nucleus will form and not decay for periods much longer than the age of the universe • A nucleus forms, but will eventually decay by transmuting itself into a different nucleus with different N and Z. “Eventually” can be anything from 10-12 sec to >1015 years. • Like atoms, nuclei can also exist in excited states, from which they ultimately decay. • Decay of unstable nuclei (‘radioactive decay’) is much more energetic than decay of excited atoms and can involve either photons or particles or both (usually the latter). • Radioactive decay must obey all conservation laws (although it is mass-energy that is conserved, not just mass or energy).
Radioactive Decay • There are 5 modes of radioactive decay • Gamma • Emission of a high energy photon; usually accompanies other kinds of decay, but can occur when an excited nucleus decays to its ground state. • Beta • Emission of a positron (β+) or electron (β–) as a proton transforms to a neutron or visa versa. In addition, a neutrino (ν) is also emitted. Its emission is necessary to conserve energy and spin (angular momentum). • Because of the nuclear structure and the way the elements were created, β- is more common that β+. • A remains unchanged but N and Z change - hence the element changes. • Electron capture • An inner electron is captured, transforming a proton to a neutron - same effect as β+ decay. X-rays emitted as outer electrons cascade inward. • Alpha • Emission of a helium nucleus or α-particle. Reduces N and Z by 2 and A by 4. Only occurs in elements heavier than Fe (and in fact 147Sm is the lightest naturally occurring nuclide that undergoes α-decay). • Spontaneous Fission • Nucleus splits into subequal parts plus free neutrons. Only occurs in very heavy nuclei. Only occurs in 238U among naturally occurring nuclei - and even then it is extremely rare. • Capture of one of these neutrons will induce fission in 235U.