Chemistry Q&A Companion
200 likes | 218 Vues
Explore chemistry questions on elements, electron configurations, ionization energy, and more. Test your knowledge in a fun and educational way.

Chemistry Q&A Companion
E N D
Presentation Transcript
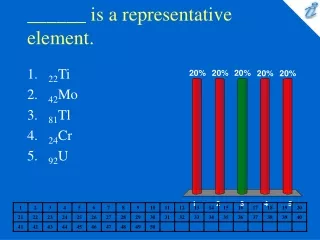
______ is a representative element. • 22Ti • 42Mo • 81Tl • 24Cr • 92U
What would be the outer electron configuration of group IVA (C, Si, Ge, . . .)? • ns2nd2np0 • ns2np4 • ns0np4 • ns1np3 • ns2np2
Choose the term that best describes all members of this series of elements: V, Cr, Fe, Co, Ni • metalloids • d-transition elements • alkaline earth metals • alkali metals • representative elements
Which element has the largest atomic radius? • Mo • Mg • Ba • Cl • At
Which element has the lowest first ionization energy? • Al • Si • P • S • Cl
Which of the following elements has the most negative electron affinity? • Ge • I • Br • Se • K
Which response includes all of the following that are isoelectronic with Kr, and no other species? I. S2- II. ArIII. K+ IV. Sr2+ V. Br- • I, II, and III • II and IV • III and IV • IV and V • I, IV, and V
Consider the following atoms and ions: Na, Na+, Mg, Mg2+, O, O2-, F, F-. Which one of the following groups includes only isoelectronic species? • Na, Mg, O, F • Na, Na+, Mg, Mg2+ • O, O2-, F, F- • Na+, Mg2+, O2-, F- • none of these
Which ion has the largest radius? • P3- • S2- • Cl- • N3- • F-
Which ion has the smallest radius? • K+ • Rb+ • Ca2+ • Sr2+ • Ba2+
Which one of the following properties is based on the attraction of an atom for electrons in a chemical bond? • binding energy • mass defect • electron affinity • ionization energy • electronegativity
Which element has the lowest electronegativity? • Be • Ba • Ga • Tl • I
Which of these comparisons of electronegativities is (are) correct? I. O > N II. S > P III. Rb > Li IV. Br > Cl • I and IV • I and III • I and II • II and III • III and IV
Which one of the following hydrides is acidic? • RbH • SrH2 • H2S • NaH • NH3
The principal product of the reaction of iron, Fe, with a limited amount of oxygen is I , while with an excess of oxygen it is II . I / II • Fe3O2 / FeO • Fe2O3 / FeO • FeO / Fe3O2 • FeO / Fe3O4 • FeO / Fe2O3
The most common oxidation states of nonmetals do not include _______ . • zero • the periodic group number of the nonmetal • the periodic group number of the nonmetal minus two • the periodic group number of the nonmetal minus eight • the negative of the periodic group number of the nonmetal
The most common oxidation states of selenium are +4 and +6 in molecular oxides. What is the formula for the product of the reaction of selenium with a limited amount of oxygen? • SeO • SeO2 • SeO3 • Se2O3 • Se3O4
The acid for which N2O3 can be considered the anhydride is __________. • H2N2O2 • HNO2 • HNO3 • H2NO3 • HN3
Write the balanced formula unit equation for the reaction of arsenic, As4, heated with a limited amount of oxygen (The molecular formula for the product is twice the empirical formula). What is the sum of the coefficients? • 2 • 8 • 3 • 4 • 5
Write the balanced formula unit equation for the reaction of tetraphosphorus decoxide with excess water. What is the sum of the coefficients? • 10 • 7 • 9 • 11 • 5