Understanding Solutions and Their Properties in AP Chemistry: Focus on CaCl2 and Concentrations
150 likes | 294 Vues
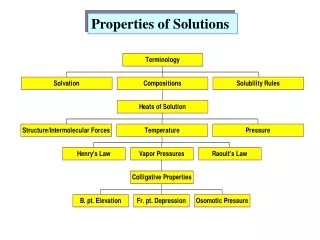
This resource explores essential concepts in AP Chemistry regarding solutions, particularly those involving CaCl2. It details when solutions form, the role of solute-solvent interactions, and the significance of disorder in mixing. Additionally, it outlines concentration expressions such as mass percent, molarity, and molality, alongside crucial vocabulary like solvation and saturation levels. Factors affecting solubility—including temperature and pressure—are examined, and colligative properties, Boiling Point Elevation, and Osmotic Pressure are highlighted to illustrate their practical implications.

Understanding Solutions and Their Properties in AP Chemistry: Focus on CaCl2 and Concentrations
E N D
Presentation Transcript
Properties of Solutions Brown, LeMay Ch 13 AP Chemistry CaCl2 (aq)
When do solutions form? • Solutions form (the solute and solvent will mix) when: • Energy:solute-solvent interactions are stronger than solute-solute or solvent-solvent interactions. • Disorder:Solutions result in a more disordered state than the separate solute and solvent states, since molecules will be “mixed” that were once “well organized”. • NaCl (s) + H2O (l) → Na+ (aq) + Cl- (aq) • Ion-dipole interactions > H-bonds (H2O···H2O) < Ionic bonds (Na+ Cl-) • The increase in disorder also drives the dissolving process.
13.2: Ways to Express Concentration • Mass Percent • Mole Fraction:commonly used for gases • Molarity:commonly used for solutions • Molality:commonly used for colligative properties • Varies with T • Does not vary with T
13.3: Solubility Vocabulary • Solvation:dissolving; the interactions between solute and solvent • Hydration: solvation with water • Crystallization: “un-dissolving”; process by which solute particles leave the solvent. • Solute + solvent ↔ solution (equilibrium)
13.3: Solubility Vocabulary • Saturated: a solution that is in equilibrium with undissolved solute (appears as solution and crystals) • Solubility: the amount of solute needed to form a saturated solution • Unsaturated:a solution containing less than the saturated amount (appears as solution only) • Supersaturated: a solution containing more than the saturated amount, yet appears unsaturated.
13.4: Factors Affecting Solubility • “Like dissolves like.” • Miscible: liquids that mix (polar or ionic solute with polar solvent, or nonpolar with nonpolar) • Immiscible:liquids that do not mix (polar or ionic solute with nonpolar solvent) • Covalent network solids do not dissolve in polar or nonpolar solvents.
13.4: Factors Affecting Solubility • Pressure: does not significantly affect solubility of liquids and solids • Gases: increased P means increased solubility Henry’s law: Cg = k Pg Cg = solubility of gas in solution (M) k = Henry’s law constant Pg = partial pressure of gas over solution William Henry(1775-1836)
13.4: Factors Affecting Solubility • Temperature • Most solids: increased T means increased solubility • * Exception: Ce2(SO4)3 • Gases: increased T means decreased solubility
13.5: Colligative Properties • Properties that are dependent on the number of solute particles present in solution • Vapor pressure lowering: the greater the concentration of a nonvolatile solute, the lower the vapor pressure of the solvent • Solute takes up surface area • Introduction of solute-solvent IMF Raoult’s law: PA = XA P°A PA = vapor pressure of solvent vapor above solution (solute A is nonvolatile) XA = mole fraction of solute A P°A= normal vapor pressure of solvent François-Marie Raoult(1830-1901)
Ideal solution:described by Raoult’s law • Has low concentration of solute • Solute and solvent have similar types of IMF & molecular sizes
Extension of the Liquid Phase • Boiling point elevation: DTb = i Kb m Kb (H2O) = 0.51 ºC•kg/mol • Freezing point depression: DTf = i Kf m Kf (H2O) = 1.86 ºC•kg/mol • i = van’t Hoff factor: Unitless constant associated with the degree of dissociation of a solute in a solvent Jacobus van’t Hoff(1852-1911)
Ideal i values • i = 1 Substances which do not ionize in solution Ex: sucrose (sugar) • i = 2 Substances which ionize into 2 ions Ex: NaCl • i = 3 Substances which ionize into 3 ions Ex: MgCl2 Ex: Determine the solute “equivalent molality” (factoring in i) for the following solutions: • 1-m sucrose • 1-m NaCl • 1-m CaCl2
P Solvent “wants” to flow Osmotic Pressure (P) • Pressure required to prevent osmosis of solute particles P = iMRT = (n/V)RT R = 0.0821 L-atm/mol-K • Applied on solution side to stop net movement of solvent from the pure solvent side. • Osmosis: net movement of solvent toward the solution with the highest solute concentration Prevents flow of solute particles
13.6: Colloids • Mixtures containing particles intermediate between: • A solution (homogeneous, < 10 Å) and • A suspension (heterogeneous, > 2000 Å) • Tyndall effect:scattering of lightseen in a colloid John Tyndall(1820-1893)