Coordination Chemistry: Introduction & Applications
330 likes | 1.06k Vues
Explore Coordination Chemistry basics with a focus on complex ions, ligands, isomers, and spectrochemical series. Discover the importance of coordination compounds in real-life examples such as hemoglobin and chlorophyll. Engage with key concepts through interactive learning resources.

Coordination Chemistry: Introduction & Applications
E N D
Presentation Transcript
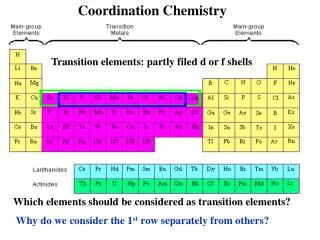
Coordination Chemistry A basic introduction with a critical application!
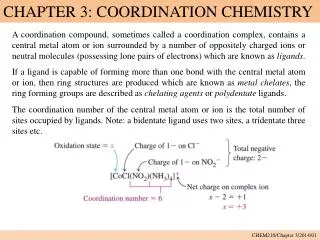
Coordination Compound • Complex ion = a transition metal with associated ligands • Counter ions = cations or anions needed to produce a neutral charge on the complex ion.
Coordination Number • Number of ligands attached to the metal Zumdahl, 5th ed.
Ligand • A neutral molecule or ion having a lone e- pair that can be used to forma a bond to a metal ion • Coordinate covalent bond = metal-ligand bond • Unidentate (monodentate) ligand = a ligand that can form only one bond to a metal ion • Bidentate= a ligand that can form two bonds to a metal ion • Chelate= a ligand that can form more than one bond to a metal ion.
Isomers Zumdahl, 5th ed.
Crystal Field Model Approximations • ligands are negative point charges • metal ligand bonding is 100% ionic. • The overlap of orbitals ___________ energy.
Octahedral Zumdahl, 5th ed.
Tetrahedral Zumdahl, 5th ed.
Degree of Overlap • Can you group the d orbitals in an octahedral complex based on their degree of overlap with the ligands? • How about in a tetrahedral complex?
Splitting patterns • Octahedral = 2 over 3 • Tetrahedral = 3 over 2
ΔE tet vs. ΔEoct • Is there a relationship between ΔEtet vs. ΔEoct? • If so, what is it? Justify your answer.
Strong vs. Weak Field • Strong field (high ∆E) = low spin • Weak field (low ∆E) = high spin Spectrochemical Series CN1- > NO21- > en > NH3 > H2O > OH1- > F- > Cl- > Br- > I-
Light Absorption & Color • Can you remember the formula we use to calculate the wavelength of light something absorbs? • What variables does it involve? • Is there more than one formula? • If so which one would be appropriate here? • How does the wavelength of light absorbed by something generally relate to its color?
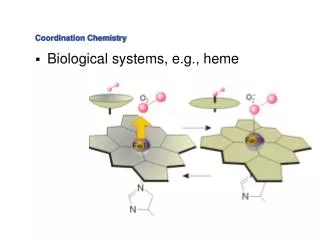
Why are coordination compounds important? • Two very good examples are hemoglobin and chlorophyll! • Can you propose from these structures and from what you have learned so far about coordination compounds what might make these compounds significant? http://www.wheatgrass.com/book/chapter3.php
Let’s focus of hemoglobin! • Hemoglobin is a protein which serves the function of binding O2 in your blood so that it can be carried to your tissues, etc. http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html
Two key components • Heme group – Fe2+ coordinated to a molecule known as a porphyrin. • Histidine residue http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html
Heme • Fe2+ complexed with the porphyrin molecule. • How would you describe the porphyrin molecule? • How many ligands does it represent? • What type of geometry and splitting pattern would you expect this to take on? http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html
Heme + Histadine = Hemoglobin • What do you expect this to do to the shape? • What type of splitting to you expect it to undergo now? http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html
Return to the function of Hemoglobin • What did we say the function of hemoglobin is? • Now that you can see the structure of hemoglobin can you predict how its structure and function relate? http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html
Let’s watch a little movie clip Click diagram to play movie! http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html
Some more pics of the oxy and deoxy hemoglobin conformations!
A little bit of anatomy! • Where is your blood oxygenated? • Where is it not oxygenated (deoxygenated)? • What color is your blood when you have it drawn for a test or when you give blood? • Where is blood drawn from when you have one of these tests? • What color are your veins?
Blue vs. Red Blood • Can we come up with a complex ion explanation for the blue vs. the red color of blood? • What questions should we ask? • Let’s define our essential question: Why is our blood red sometimes and blue others?
Key Questions • Why does something appear a particular color? • How do color absorbed and color reflected relate? • What color is the red blood absorbing? • What color is the blue blood absorbing? • Draw plots of the absorption spectrum you would expect for oxyhemoglobin and deoxyhemoglobin.
Color and Coordination • We know hemoglobin is a coordination compound and we are investigating its color. • How do coordination compounds and color relate? • Can you define the relationship we need to use in order to relate them?
Energy and Color • How does the color of light absorbed relate to the energy of the complex? • Using grammatically correct English sentences compare the energy of deoxyhemoglobin to that of oxyhemoglobin base on their respective colors.
Splitting Patterns and Color • What does the difference in color tell us about the splitting patterns in deoxy- and oxyhemoglobin respectively? • Do you expect them to have different splitting patterns all together? Justify your answer.
HOMEWORK • Write a one page explanation of the difference in color between oxy- and deoxyhemoglobin on the basis of coordination complexes. • Be sure your explanation is understandable to any of your classmates who have taken chemistry at ANY level.
References • Zumdahl, Chemistry, 5th ed. • http://www.chemistry.wustl.edu/~edudev/LabTutorials/Hemoglobin/MetalComplexinBlood.html