The Gas Laws Section 14.1
60 likes | 215 Vues
This section explores the behavior of ideal gases through the lens of Boyle’s Law, Charles’s Law, and Gay-Lussac’s Law. It explains how pressure, volume, and temperature interact, illustrating fundamental concepts through practical examples and laboratory applications. We review key assumptions of the Kinetic Molecular Theory (KMT), discussing molecular motion and energy during collisions, as well as comparing average kinetic energy of gases at the same temperature. Engage in exercises to reinforce understanding of these gas laws.

The Gas Laws Section 14.1
E N D
Presentation Transcript
The Gas Laws Section 14.1 Objectives: 7.0 Explain the behavior of ideal gases in terms of pressure, volume, and temperature using Charles's law, Boyle's law, and Gay-Lussac's law.
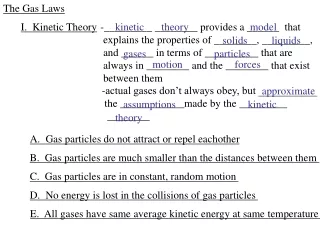
KMT Review 3 Assumptions: Intermolecular forces? Size and space? Motion? What happens to the kinetic energy when gas particles collide? If two gases have the same temperature, how does the average kinetic energy of the gases compare?
Boyle’s Law Lab • Boyle’s law states that the ________ of a gas varies ___________ with _____________. • This means that as volume increases, pressure ______________. • Boyle’s Law: P1V1 = P2V2 • Give an example of application of Boyle’s law. • Example/Practice: P.422
Charles’s Law • What happens to a balloon if you put it in a warm car? • So how are temperature and volume related? • As temperature increases, volume _________. • Charles’s Law: V1/T1 = V2/T2 • What units should you use for temperature in this equation????? • Example/Practice: P.425
Gay-Lussac’s Law • Think about the balloon again. What happens to volume if temperature increases? So what will happen to the pressure if temperature increases? • Gay-Lussac’s Law: P1/T1 = P2/T2 • Again, what units MUST temperature be in? • Example/Practice: P.427
Assignment • Workbook: P.79 • P.448: 88-91