formation
20 likes | 197 Vues
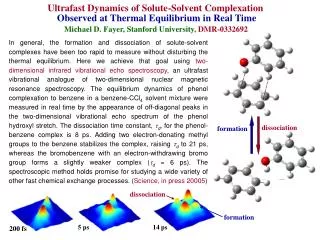
dissociation. formation. dissociation. formation. 5 ps. 14 ps. 200 fs. Ultrafast Dynamics of Solute-Solvent Complexation Observed at Thermal Equilibrium in Real Time Michael D. Fayer, Stanford University , DMR -0332692.

formation
E N D
Presentation Transcript
dissociation formation dissociation formation 5 ps 14 ps 200 fs Ultrafast Dynamics of Solute-Solvent Complexation Observed at Thermal Equilibrium in Real TimeMichael D. Fayer, Stanford University,DMR-0332692 In general, the formation and dissociation of solute-solvent complexes have been too rapid to measure without disturbing the thermal equilibrium. Here we achieve that goal using two-dimensional infrared vibrational echo spectroscopy, an ultrafast vibrational analogue of two-dimensional nuclear magnetic resonance spectroscopy. The equilibrium dynamics of phenol complexation to benzene in a benzene-CCl4 solvent mixture were measured in real time by the appearance of off-diagonal peaks in the two-dimensional vibrational echo spectrum of the phenol hydroxyl stretch. The dissociation time constant, d, for the phenol-benzene complex is 8 ps. Adding two electron-donating methyl groups to the benzene stabilizes the complex, raising d to 21 ps, whereas the bromobenzene with an electron-withdrawing bromo group forms a slightly weaker complex (d = 6 ps). The spectroscopic method holds promise for studying a wide variety of other fast chemical exchange processes. (Science, in press 20005)
Ultrafast Dynamics of Solute-Solvent Complexation Observed at Thermal Equilibrium in Real TimeMichael D. Fayer, Stanford University,DMR-0332692 Outreach: The PI is participating in an NSF sponsored program to bring high school science teachers into the research laboratory over the summer. Rachel Gaffuri teaches physics and chemistry at Mt. Eden High in Union City, California. She is performing research on nanoscopic water in reverse micelles. Education: A postdoc(John Asbury)and three graduate students (Ivan Piletic, Junrong Zheng, and Kyungwon Kwak) contributed to this work. John Asbury has just started as an Assistant Professor in the Department of Chemistry at Pennsylvania State University where he will be conducting semiconductor materials research. Ivan Piletic is an advanced graduate student who will be completing his Ph.D. in chemistry in approximately one year. He will do a post doc and is headed toward academia. Junrong Zheng and Kyungwon Kwak have just completed their second year of the Ph.D program. They are superb students who are performing experiment and theory far beyond what one would expect given the length of time they have been at Stanford.