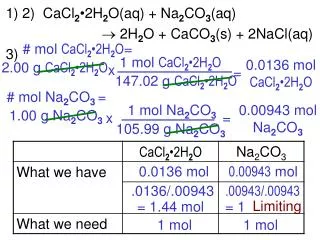
1 mol - PowerPoint PPT Presentation
View 1 mol PowerPoint (PPT) presentations online in SlideServe. SlideServe has a very huge collection of 1 mol PowerPoint presentations. You can view or download 1 mol presentations for your school assignment or business presentation. Browse for the presentations on every topic that you want.