Dynamics of Chemical Reactions and Reactants Transformation
130 likes | 154 Vues
Analyzing reaction rates and intermediates in various chemical reactions to understand the kinetics and mechanisms involved. Calculations, graphs, and rate determinations covered in Chapter 14 topics.

Dynamics of Chemical Reactions and Reactants Transformation
E N D
Presentation Transcript
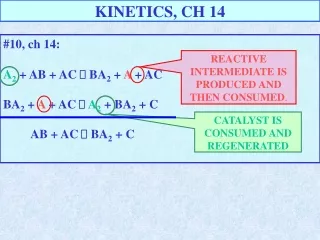
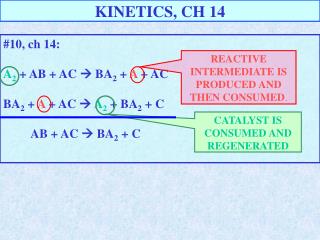
KINETICS, CH 14 #10, ch 14: A2 + AB + AC BA2 + A + AC BA2 + A + AC A2 + BA2 + C AB + AC BA2 + C REACTIVE INTERMEDIATE IS PRODUCED AND THEN CONSUMED. CATALYST IS CONSUMED AND REGENERATED
#16 SLOPE OF LINES GIVES RATES: AT 75 MINRATE IS 4.2 * 10-5 M/min. OR 7.0 * 10-5 M/sec AT 250 MIN RATE IS 2.1 * 10-3 M/min (3.5 * 10-3 M/sec )
#20, ch 14: 2 THE C2H4 DISSAPEARS AT A RATE OF 0.37 M/s, AND WATER AND CO2 APPEAR TWICE AS FAST 2(0.37 M/s) = 0.74 M/s. [CO2]/2 t [H2O]/ t - [C2H4]/ t - [C2H4]/ t [H2O]/2 t = = [CO2]/ t = = INDICATES C2H4 NEEDS TWICE THE TIME TO DISSAPEAR AS IT TAKES FOR CO2 AND H2O TO APPEAR. INDICATES THE CO2 AND H2O APPEAR IN ½ THE TIME (TWICE AS FAST) AS IT TAKES THEC2H4 TO DISSAPEAR. C2H4 +3O2 2CO2 + 2 H2O N2H4 +H2 2NH3 N2H4 IS CONSUMED AT AT RATE OF 63 TORR/hr, THUS NH3 IS PRODUCES AT DOUBLE THAT RATE: 2(63 TORR/hr) = 126 TORR/hr
#32 CH 14 S2O82-+3 I- 2 SO42-+ I3- [S2O82-] INCRESES A FACTOR OF 1.5 WHILE THE RATE INCRESES BY 1.5: S2O82- IS FIRST ORDER.
#32 CH 14 S2O82-+3 I- 2 SO42-+ I3- [S2O82-] INCRESES A FACTOR OF 2, I- BY A FACTOR OF1.5, WHILE THE RATE INCRESES BY 3 FOLD: S2O82- IS FIRST ORDER(KNOWN) AND I- MUST BE FIRST ORDER. 2 * 1.5 = 3 : 31 = 3 The exponent of 1 makes the equation true, the reactants are first order
#32-B S2O82-+3 I- 2 SO42-+ I3- RATE=k [S2O82-][I-] ,VALUES FROM TABLE SUBSTITUTED IN. AVERAGE k = 4.0 * 10-3 M-1 s-1 #32-C DISSAPEARS 1/3 AS RAPIDLY AS I- , THEREFORE RATE = 3 (4.0 * 10-3)(0.015 M )(0.040 M) = 7.2 * 10-6 M/s = RATE OF I- DISSAPEARANCE S2O82-
#42 CH 14 Plot of ln A vs t is linear,Reaction is 1st order in A 1/mol(A) ln(A) t
#42 CH 14-b 42-b k = -slope k = -[-3.91 –(-2.70)]/120 = 0.0101 s-1 Plot of ln A vs t is linear,Reaction is 1st order in A ln A 42-c t1/2 = 0.693/k = 0.693/0.0101 = 68.7s ln A
#48-a CH 14 f =e-Ea/RT a) -Ea/RT = 1.60 J/mol x mol*K = -38.489 500K8.314 J f =e-38.489 = 1.92 * 10-17 b) -Ea/RT = 1.60 J/mol x mol*K = -37.735 510K8.314 J f =e-37.735 = 4.09 * 10-17 4.09 * 10-17 / 1.92 * 10-17 = 2.13 times more at 510K
#54 CH 14-b ln [k1/k2]= Ea/R [1/T2 – 1/T1] ln [0.0796/0.0815]= Ea/8.314J/mol [1/1220 – 1/1010] -0.023589 = Ea (-1.704 * 10-4) / 8.313 J/mol Ea = 8.313(-0.023589)J/mol = 1.151 * 103 J/mol
#68 CH 14 • HBr + O2 HOOBr (SLOW) • HOOBr + HBr 2 HOBr • 2 HOBr + 2 HBr 2 H2O + 2 Br2 • 4 HBr + O2 2H2O + Br2 b) GIVEN VERBALLY IN PROBLEM RATE = [HBr][O2], BOTH FIRST ORDER. THIS AGGREES WITH STEP 1) WHICH MUST BE SLOW. c) HOOBr AND HOBr ARE INTERMEDIATES d) THE INTERMEDIATES MY NOT ACCUMULATE IF THEY ARE IN A FAST STEP AFTER A SLOW ONE AS HERE.
#72 CH 14 • 2 [ N2O + NO N2 + NO2] • 2 NO2 2 NO + O2 • 2 N2O 2N2 + O2 INTERMEDIATE CATALYST • 2 N2O + 2 NO 2 N2 + 2 NO2 (SLOW) • 2 NO2 2 NO + O2 • 2 N2O 2N2 + O2 THE INTERMEDIATE NO2 DOES NOT ACCUMULATE AS IT IS PRODUCED IN THE SKOW STEP AND CONSUMED IN THE FAST STEP
#80 CH 14 ln kc – ln k = [ Ea – Eac/RT] or Ea-Eac = RT ln (kc/k) Where c is with catalyst. Represents the ratio of rates asked for in problem. Ea-Eac = RT ln (kc/k) Ea-Eac =8.314 J * 310K * ln (1 * 105) Ea-Eac =2.966 * 104 = 30. kJ