Stereochemistry
490 likes | 1.24k Vues
Stereochemistry. Paderborn, May 2005. Founding Fathers of Stereochemistry. Biot. Biot : The solutions of many naturally occurring compounds rotate the plane of polarization of polarized light (1815-1817)

Stereochemistry
E N D
Presentation Transcript
Stereochemistry Paderborn, May 2005
Founding Fathers of Stereochemistry Biot • Biot: The solutions of many naturally occurring compounds rotate the plane of polarization of polarized light (1815-1817) • Pasteur recognized in 1850 that this optical activity was caused by an asymmetric arrangement of atoms in a molecule • van’t Hoff and Le Bel described in 1874 how the atoms of a molecule are actually arranged in space Pasteur Van’t Hoff
Subdisciplines of Stereochemistry • Static stereochemistry • Studies the three-dimensional arrangement of the atoms of a molecule in the ground state • Dynamic stereochemistry • Description of the steric relationships in molecules as they change from one state to another, for example during a chemical reaction
Preview • Introduction • Conformational analysis • Cyclohexane • Bicyclic compounds, steroids • Heterocyclic compounds • Optical activity and stereoisomerism • Symmetry and chirality • Molecular asymmetry • Prochirality • Chiroptical properties of chiral molecules • Optical rotatory dispersion
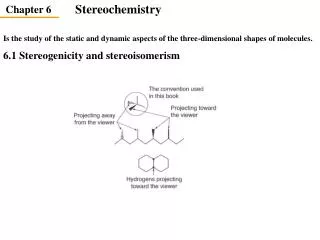
Introduction • Structure: Includes bothconstitution and configuration. • Constitution: Describes the kinds and order of the bonds and atoms or atom groups in a compound. • Configuration: Describes the different spatial arrangements of atoms or atom groups of a compound with a given constitution. • Stereoisomerism • Enantiomers: Image and mirror image are not identical • Diastereomers: Stereoisomers that are not mirror images • Conformation:Describes the different spatial arrangements of atoms or groups in a molecule that arise due to rotation (torsion) around single bonds.
Examples • Structure and Constitution: • Configuration:
Examples • Configuration: • Stereoisomerism • Enantiomers: Image and mirror image are not identical • Diastereomers: Stereoisomers that are not mirror images
Conformational Analysis • Cyclohexane • Bicyclic systems and steroids • Heterocyclic systems
Optical activity and Stereoisomerism • Symmetry und chirality • Symmetry axis Cn • Symmetry plane σ • Symmetry centre i • Rotation/reflection axis Sn • Molecular asymmetry • Chiral axis • Chiral plane • Chiral centre • Prochirality
Symmetry and Chirality • n–Fold axis of symmetry Cn • Plane of symmetry σ
Symmetry and Chirality • Centre of symmetryi • n-Fold rotation-reflection axis Sn
Symmetry and Chirality • Molecules with no reflection symmetry are chiral • A molecule with only a Cn axis is chiral
Molecular Asymmetry • Chiral axis • Chiral plane • Chiral centre
Chiral Plane 1 a b c 2 3 R • Lead atom: atom with highest priority directly linked to the plane • Determine the atom sequence in the plane • Determine chirality, starting from the lead atom
Prochirality • Enantiotopos • Enantiofaces • Diastereotopos • Diastereofaces
Heterotopy • Homotopic • Heterotopic • Constitutopic • Stereoheterotopic • Enantiotopic • Diastereotopic
Substitution Test • Identical molecules • Homotopic (equivalent) • Isomers • Heterotopic • Constitutional isomers • Constitutopic • Stereoisomers • Stereoheterotopic • Enantiomers • Enantiotopic • Diastereomers • Diastereotopic
Chiroptical Properties of Chiral Molecules • A linearly polarized wave may be described as the result of a left polarized wave superimposed on a right polarized wave • Left and right polarized waves are absorbed differently by an optically active compound • When the two components are recombined after passing through an optically active medium, the result is an elliptically polarized wave with ellipticity θ:
Optical Activity • Optically active compounds are circularly birefringent – the refractive indices of the left and right polarized waves differ: • v = c/n , therefore, if vL ‡ vR , then nL ‡ nR • There is a phase difference, resulting in optical rotation: • = .d(nL - nR)/ = 180d(nL - nR)/ • The optical rotation is dependent on the wavelength – Optical rotatory dispersion
Chiroptical Properties of Chiral Molecules • Optical rotatory dispersion • Plain curves • Anomalous curves
Chiroptical Properties of Chiral Molecules • Optical rotatory dispersion • Achiral chromophores • Chiral chromophores
Achiral Chromophores Achiral disturbance Chiral disturbance
Chiroptical Properties of Chiral Molecules • Optical rotatory dispersion • Constitution • Configuration • Conformation
Plain Curves • With small amounts of substance, one can measure at shorter wavelengths • To determine whether a substance is really optically active and not racemic
Chiroptical Properties of Chiral Molecules • Optical rotatory dispersion • Constitution • Configuration and conformation
The Octant Rule for Ketones 5 4 6 3 1 2
Chiroptical Properties of Chiral Molecules • Octant rule: • Configuration • Conformation • Absolute configuration
Summary • Introduction • Conformational analysis • Cyclohexane • Bicyclic compounds, steroids • Heterocyclic compounds • Optical activity and stereoisomerism • Symmetry and chirality • Molecular asymmetry • Prochirality • Chiroptical properties of chiral molecules • Optical rotatory dispersion