Atoms
120 likes | 258 Vues
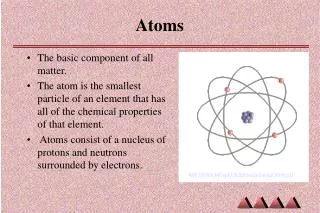
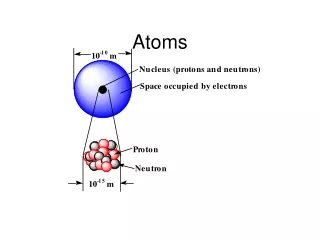
Atoms. What kind of particles make up an atom? a. protons, electrons, & neutrons b. elements and protons c. neutrons, elements, & proteins d. cells, elements, and molecules. Atoms are made of smaller (subatomic) particles. Which of these subatomic particles has the least mass? protons

Atoms
E N D
Presentation Transcript
What kind of particles make up an atom? a. protons, electrons, & neutrons b. elements and protons c. neutrons, elements, & proteins d. cells, elements, and molecules
Atoms are made of smaller (subatomic) particles. Which of these subatomic particles has the least mass? protons b. neutrons c. electrons
When an electron from one atom is transferred to a different atom, which of the following may occur: a. neither resulting atom is stable and both are destroyed b. one atom becomes a positive ion, the other becomes a negative ion and an ionic bond may form between the two c. the atoms regain their neutral status and form a covalent bond d. each atom becomes a new element
A sodium atom (Na) loses an electron. Which of the following is true? • It becomes an atom of Neon (Ne). b. It becomes an atom of Magnesium (Mg). c. It becomes a Na+ ion. d. It becomes a Na- ion.
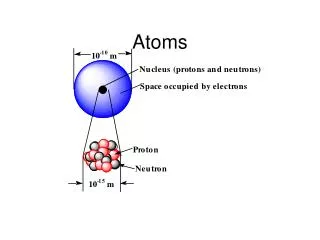
Atoms are made up of subatomic particles including protons, neutrons, and electrons; but most of the atom is empty space. • True b. False Hydrogen atom
An ion is • the resulting particle when an atom gains or loses an electron b. a group of atoms c. part of a molecule
When two atoms share an electron it is called • an ionic bond b. a covalent bond c. a hydrogen bond
An atom of Carbon has . . .Hint – Check Periodic Table(last slide) • 6 protons and 6 electrons • a mass of 3 AMUs • 12 electrons • 6 neutrons and 12 electrons
What are isotopes? a. atoms with the same number of protons but different numbers of neutrons b. atoms with different numbers of protons but the same number of neutrons c. atoms with the same number of protons but different numbers of electrons d. atoms with different numbers of electrons and different numbers of protons
Atoms have equal numbers of protons and electrons. • True • False