Unit 3: Atomic Structure
1.13k likes | 2.14k Vues
Unit 3: Atomic Structure. Featuring these scientists and more!. Castagno Chemistry Challenge III Unit 3 – Day 1. Rules: 1) You are working as a CLASS and as individual groups so you can whisper amongst the table but if you’re too loud you’ll be helping out your opponents!

Unit 3: Atomic Structure
E N D
Presentation Transcript
Unit 3: Atomic Structure Featuring these scientists and more!
Castagno Chemistry Challenge IIIUnit 3 – Day 1 • Rules: • 1) You are working as a CLASS and as individual groups so you can whisper amongst the table but if you’re too loud you’ll be helping out your opponents! • 2) You will have 6min 10s, the clock will be on the board! • 3) Credit only goes to COMPLETELY correct answers • POINTS • Class:1st – 2pts, 2nd – 1pt, 3rd – 0pts, 4th – 0pts • Group: 1st – 1pt bonus • Questions?
Castagno Chemistry Challenge IIIUnit 3 – Day 1 • You will be presented with a wordsearch without a word bank. • There are 37 terms. • 6mins 10s allows you 10seconds for each word! • The Challenge: • Find as many as you can.
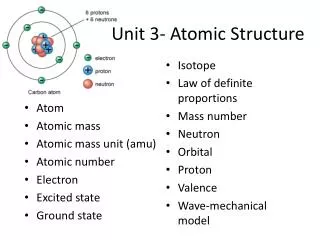
Unit 3 Objectives • Definitions: atom, proton, neutron, electron, atomic theory, electron configuration, atomic number, mass number, atomic mass unit, isotope, Avogadro’s number. • The arrangement and behavior of subatomic particles according to modern theory. • The history of atomic theory from Democritus to modern quantum theory (Bohr/Schrodinger). • The experiments that led to the discoveries of the subatomic particles. • How to use the aufbau principle, Pauli’s exclusion principle, and Hund’s rule to arrange electrons in atoms. • That atoms can gain energy and then emit it in the form of visible light. • The color (wavelength) of this light can be used for identification purposes. • The energy levels and sublevels in an atom. • The difference between atomic number and mass number in calculation as well as purpose. • How to connect atomic mass and atomic weight. • How to calculate atomic mass when given isotopes. • Avogadro’s number is 6.02 x 1023.
Essential Questions • What is the modern description of an atom? • How are atoms counted? • GUIDING QUESTIONS: • What does an atom look like? • What are the components and features of an atom? • What are the atomic theories proposed by Democritus, Dalton, and Bohr? • What are the main points in the modern atomic theory? • Who ran the experiments that discovered the subatomic particles? • How can light be used to describe the behavior of atoms? • What are the properties and locations of electrons, protons, and neutrons? • How can the arrangement and location of electrons in atoms be expressed? • What are the aufbau principle, Pauli’s exclusion principle, and Hund’s rule? • How is electron configuration and orbital notation written for an element? • What is an energy level and what is a sublevel? • How does an atomic number differ from a mass number? • How and why was the atomic mass unit established? • What is an isotope? • What is Avogadro’s number?
Your Take • Atoms in the world? P1 • Lasers • How do we see atoms? • Nuclear weapons • Radiation • Time travel • Do we touch anything? (p4) • What makes things different colors? • What makes light bright? • Atoms in a vacuum (P6) • Magnification needed to see an atom • Plum pudding? • Atomic theory/models • How an atom was discovered? • How small are atoms? • How do glow sticks/glow in the dark stuff work (P7) • Radioactive rocks • Atoms in the body
Castagno Chemistry Challenge IIIUnit 3 – Day 2 • Rules: • 1) You are working as a CLASS and as individual groups so you can whisper amongst the table but if you’re too loud you’ll be helping out your opponents! • 2) You will have 5 minutes, the clock will be on the board! • 3) Credit only goes to COMPLETELY correct answers • POINTS • Class:1st – 2pts, 2nd – 1pt, 3rd – 0pts, 4th – 0pts • Group: 1st – 1pt bonus • Questions?
Castagno Chemistry Challenge IIIUnit 3 – Day 2 • You will be presented with a crossword. • You will be presented with the wordsearch from the first Challenge of the unit. • There are 37 terms. • The Challenge: • Fill out as many as possible
Preassessment • 1) Match the scientist with his atomic model: • Bohr a) “Plum pudding” • Aristotle b) “Solar system” • Thomson c) “Infinitely divisible” • 2) What is the electron configuration of sulfur? • 3) How many protons, neutrons, and electrons can be found in a Zinc-66 atom? • 4) Draw what an atom looks like.
The Atom as Ancient History* • The following laws • Conservation of Matter • Definite Proportion • Multiple Proportion • Were derived from observations on the behavior of matter. • Interestingly these were formulated BEFORE science knew what the atom was even composed of!
Atomos the Greek God of Atoms • The Greeks were the first to discuss what “stuff” was made of. • Democritus • Leucippus • Epicurus • Lucretius Zeus preparing to slay the n00b god.
Democritus II • First (or generally credited as ‘first’) to theorize that matter was not infinitely divisible. • He called the smallest possible part of matter “atomos” • So, what did they look like?
Democritus II • Atoms of matter reflected general properties • Iron atoms = strong and solid • Water ‘atoms’ = smooth and fluid • So the first diagram of an atom
Where We Stand #1 • The history of the atom is a long one, so at the beginning of our journey, this is what is believed • 1) Democritus (~400BC) – “atomos” The Atom
Ancient Street Brawl • There’s a good reason you haven’t heard of Democritus before. • Aristotle 384BC – 322BC • He did not believe Democritus (or other atomists) theories.
Ancient Street Brawl II • His ‘theory’ can be summarized as • No atoms…no ‘substances’ even, but principles • His popularity and fame (or the fact the people didn’t think for themselves) set chemistry back 2000 years
Where We Stand #2 • 1) Democritus – ~400BC, “atomos” • 2) Aristotle – ~360BC, “principles” The Atom
From the darkness, a hero rises • After 2000 years and alchemists attempting to turn urine (among other things) into gold, John Dalton comes to the rescue Dalton was colorblind so even if this engraving was in color, it would still look the same to him.
Teacher, Quaker, Weatherman, Father • Finally, someone was going to stand up to Aristotle and use actual science! • This is why Dalton is known as the “Father of Atomic Theory.”
Dalton’s Atomic Theory Point 1 • All matter is composed of extremely small particles called atoms. • Of course this wasn’t Dalton’s idea at all, but Democritus’
Dalton’s Atomic Theory Point 2 • All atoms of a given element are identical, having the same size, mass, and chemical properties. Atoms of a specific element are different from those of any other element.
Dalton’s Atomic Theory Point 3 • Atoms cannot be created, divided into smaller particles, or destroyed. • Doesn’t this sound familiar? • Law of Conservation of Matter!
Dalton’s Atomic Theory Point 4 • Different atoms combine in simple whole-number ratios to form compounds. • This is an example of the Law of Multiple Proportions. • It is why water is H2O and not H2.5O1.2
Dalton’s Atomic Theory Point 5 • In a chemical reaction, atoms are separated, combined, or rearranged. • Doesn’t this sound familiar? • Law of Conservation of Matter!
Dalton’s Summary • Essentially he took the ideas of the time and, noticing a trend, compiled them into a theory.
Where We Stand #3 • 1) Democritus – ~400BC, “atomos” • 2) Aristotle – ~360BC, “principles” • 3) Dalton – ~1808 AD, first atomic theory The Atom
Back Where We Started • Dalton’s work just puts as back at the beginning of the story.
Breaking Down the Atom • We bravely leave the world of chemistry to journey to the world of physics. • J.J. Thomson conducted the ‘Cathode Ray Tube’ experiment.
Thomson’s CRT Experiment • http://youtu.be/XU8nMKkzbT8 • Or • http://www.youtube.com/watch?v=Rb6MguN0Uj4
What It All Means • Thomson concluded that the green was not light but particles.
What It All Means II • The particles were affected by magnets and other fields so they had a charge.
What It All Means III • A simple mass-to-charge ratio calculation resulted in a particle mass SMALLER THAN HYDROGEN!
CRT Conclusion • Thomson concluded that the particles, which he called “corpuscles,” were • A) Negative • B) Smaller than hydrogen
HOLD ON A MINUTE • If matter is neutral, how does something exist that is purely charged? The Atom
HOLD ON ANOTHER MINUTE • If the atom looks like this then how can something exist that is smaller than it? The Atom
Save Room for Dessert • Dalton’s “indivisible” atom is incorrect. • Matter is still neutral so Thomson’s final conclusion must satisfy both facts.
Hmmmmm plum puddddddddding • Thomson’s atom included electrons (corpuscle is a silly name, they said) set within a positively charged “pudding” • His atomic model is called the “plum pudding” model.
Where We Stand #4 • 2) Aristotle – ~360BC, “principles” • 3) Dalton – ~1808 AD first atomic theory • 4) Thomson – 1897 AD, “plum pudding”
Going for the Gold • Thomson won a Nobel Prize for his efforts but we know that the structure of the atom is much different than his model.
Ernest (East) Rutherford • The next major step in understanding the structure of the atom involved using a nuclear radiation source and gold… • Sounds unsafe and expensive so let’s replicate it!
Geiger-Marsden…nah, Rutherford • The “Gold Foil Experiment” by any other name… • Video: http://youtu.be/XBqHkraf8iE • Or • http://www.youtube.com/watch?v=dNp-vP17asI
Gold Medal Conclusion I • According to Thomson’s model, the alpha particles should pass through the atom uninhibited. • However, the experiment showed that some particles were deflected.
Gold Medal Conclusion II • Roughly 1 in 8000 particles were deflected • Rutherford concluded that • A dense bundle of mass is present • He called it the “nucleus”
Gold Medal Conclusion III*** • Although he was correct that the nucleus was positive, he was unaware at the time that it consisted of positive (protons) and neutral (neutrons) particles. • 1911 – discovery of nucleus • 1920 – existence of protons • 1921 – theorized existence of neutrons which was proved in 1935 • Ironically, Rutherford (who won the Nobel Prize in Chemistry in 1908) did not win the Nobel Prize in Physics for this work but James Chadwick did for proving the existence of the neutron.
Where We Stand #5 • 3) Dalton – ~1808 AD first atomic theory • 4) Thomson – 1897 AD, “plum pudding” • 5) Rutherford - ~1911 AD, “nuclear atom” The Atom
Ring Around the Atom…Pocket’s full of…Atoms. • Most of your drawings of an atoms had the electrons on some sort of ring around the nucleus…
Well This Sure Isn’t Bohring • The first real insight into the behavior of an atom came from the study of hydrogen • It is the smallest atom (1 proton and 1 electron) and therefore the easiest to study
Hydrogen Emission Spectrum • Niels Bohr was the first to describe the behavior of an atom mathematically • Video: http://www.youtube.com/watch?v=QI50GBUJ48s
Bohr and his Model • As the video showed, this is the model he developed • Electrons can exist on a ring, move from one to the other, but never inbetween.