½ sheet Quiz 2.5
800 likes | 1.01k Vues
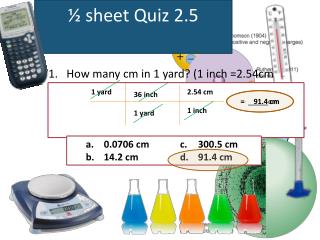
½ sheet Quiz 2.5. 1 yard. 2.54 cm. 36 inch. = _____cm. = 91.4 cm. 1 inch. 1 yard. How many cm in 1 yard? (1 inch =2.54cm). 0.0706 cm 14.2 cm 300.5 cm 91.4 cm. ½ sheet Quiz 3.1. What is the word, meaning Indivisible in Greek?. ATOM. Atoms. Building Blocks of Matter.

½ sheet Quiz 2.5
E N D
Presentation Transcript
½ sheet Quiz 2.5 1 yard 2.54 cm 36 inch = _____cm = 91.4 cm 1 inch 1 yard How many cm in 1 yard? (1 inch =2.54cm) • 0.0706 cm • 14.2 cm • 300.5 cm • 91.4 cm
½ sheet Quiz 3.1 • What is the word, meaning Indivisible in Greek? ATOM
Atoms Building Blocks of Matter The atom song
The dawn of the discovery of atomic structure • Democritus- first used term atom • Meant indivisible in Greek • Aristotle- did not believe in atoms • This opinion was accepted as fact for nearly 2000 years • No evidence for either idea
Evidence becomes available • Law of conservation of mass Mass is neither created nor destroyed during ordinary chemical reactions
Evidence becomes available • Law of definite proportions The same compound contains elements in the same ratio, regardless of the amount of material Example: water is H2O, 2 hydrogen for each 1 oxygen
Evidence becomes available • Law of multiple proportions If two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers Example: 16 g of oxygen reacts with 2 g of hydrogen to form water
Evidence Becomes Available • Law of multiple proportions 14 g of N bond with 16 g of O to form NO 14 g of N bond with 32 g of O to form NO2
Can you… • State and explain the laws of • Conservation of mass? • Definite proportions? • Multiple proportions?
Dalton’s Big Idea • All matter is composed of atoms • Atoms of the same element are identical to each other • Atoms cannot be broken down, created or destroyed • Atoms combine together in whole number ratios • Atoms are rearranged in chemical reactions in ordinary reactions!
Can you… • Summarize the five essential points of Dalton’s atomic theory? • Explain the relationship between Dalton’s atomic theory and the laws of Conservation of mass? Definite proportions? Multiple proportions?
Classwork/Homework 3.1 • CW 3.1 page 6 of handout
Dalton’s Big Idea 1 point each. Total of 5 ½ sheet Quiz 3.1 • All _______is composed of atoms • Atoms of the same _______are identical to each other • Atoms cannot be broken down, ________ or __________in ordinary reactions! • Atoms combine together in small, whole number ratios. • _____are rearranged in chemical reactions matter element created destroyed Atoms CB ____________
Previously believed… • Matter can be broken down to atoms, but no further
Evidence dispels prior beliefs • Thomson discovers the electron • Uses the cathode ray
How we know something is there How we know cathode rays normally travel straight
How we know there are particles How we know the particles are negatively charged
The electron is discovered • JJ Thomson and the cathode ray Established that there were negative particles within atoms Established that the negative particles made up very little of the mass of an atom Inferred that there were positive components to atoms Mass of the electron is 9.109 x 10-31 kg
“Plum Pudding” • Used to describe Thomson’s view of the atom • Negative particles are suspended in a positive background (modern: think mint chip ice cream)
Rutherford “The nucleus is discovered” • The experimental set-up
The nucleus is discovered • What was expected to happen • All alpha particles would pass through the atom unimpeded α particles
The nucleus is discovered • What did happen • Most alpha particles passed through the atom unimpeded • Some bounced back α particles
Explaining the results • Occasionally the α-particles were hitting something relatively large and solid • Video1 Video2
Try it Yourself! • Can you figure out the shape of the target?
Try it Yourself! • Can you figure out the shape of the target?
Can you… • Summarize the observations that led to the discovery of the electron? • Describe Rutherford’s gold foil experiment?
QUARKS equal in a neutral atom Atomic Number equals the # of... Most of the atom’s mass. Subatomic Particles ATOM NUCLEUS ELECTRONS NEUTRONS PROTONS NEGATIVE CHARGE POSITIVE CHARGE NEUTRAL CHARGE
Can you compare and contrast? Found in nucleus Have a charge Mass number =1 Make up atoms Different members of the same element may vary in number
Can you… • List the properties of protons, neutrons and electrons? • Define atom?
How do we describe an atom of an element? • Atomic notation • Periodic table notation
Thursday Homework • HW 3.1 Complete the LAB Packet pg 6 Text page 71 (1-3), page 89 (1-2) • Homework 3.2 Page 7-8 Packet Text page 76(1-5) Page 89 (3-6)
June 7th ½ sheet 3.2 1 point each. Total of 9 List the 3 following properties of protons , neutrons & electrons. Location: _______ ________ ________ Mass: _______ ________ ________ Charge: _______ ________ ________ nucleus Orbit or cloud nucleus 0 1 1 neutral -1 +1 Notes are on PAGE 4
Most of the atom’s mass. Subatomic Particles ATOM NUCLEUS ELECTRONS NEUTRONS PROTONS NEGATIVE CHARGE POSITIVE CHARGE NEUTRAL CHARGE
How do we describe an atom of an element? • Protons • Atomic number • Number of protons • NEVER changes for a given element • Defines the identity and characteristics of the atom • If it is carbon it has 6 protons • If it has 6 protons it is carbon (Z) • A hydrogen atom A carbon atom A gold atom • (1 proton) (6 protons) (79 protons)
How do we describe an atom of an element? • Electrons • For atoms, the number of electrons is equal to the number of protons (the atomic number) • They will differ in ions (later in semester)
How do we describe an atom of an element? • Neutrons • Atomic mass or Mass number • Number of protons + number of neutrons • May change for a given element (isotopes) A -Z n0 (A) mass -protons neutrons
A -Z n0 mass -protons neutrons Mass Number Practice Mass number is the number of protons and neutrons in the nucleus of an isotope. Mass # = p+ + η0 18 8 8 18 Arsenic -75 33 75 Phosphorus 16 15 31
Designating Isotopes page 4 0 1 1 1 1 1 Hydrogen-3 1 6 6 6 6 6 8 Carbon-14 9 Fluorine-19 9
Open worksheets to Page 7 • Complete Pages 7 and 8 with your Z buddy
How do atoms of the same element differ? • Isotopes • Carbon-12 has 6 p+ and 6η0 • Carbon-14 has 6 p+ and 8η0 Class notes page 4
Isotopes • Can be written 3 ways • Uranium mass= 238 Uranium-238 (U-238) • Oxygen with 7 neutrons Oxygen-15 (O-15)
Upcoming Challenge • Doc Oc says there is 25 lbs of tritium on Earth? How many atoms is that? (This number is not correct)
Can you… • Describe an isotope? • Define atomic number? • Define mass number? • Determine the numbers of protons, neutrons and electrons in an atom given atomic notation?