Structure and Function of Subatomic Particles
70 likes | 278 Vues
Structure and Function of Subatomic Particles. *The identity of an atom is determined by the number of it contains. *The number of in an atom is referred to as the atom’s .

Structure and Function of Subatomic Particles
E N D
Presentation Transcript
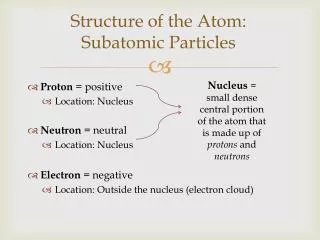
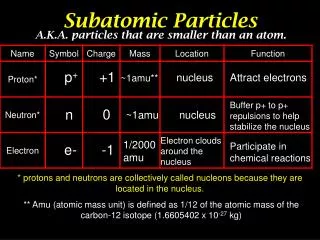
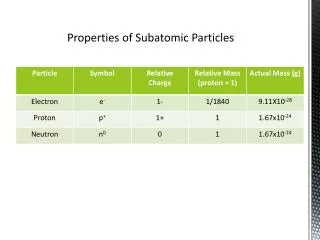
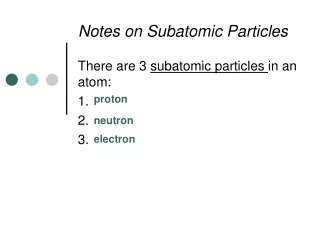
*The identity of an atom is determined by the number of it contains. *The number of in an atom is referred to as the atom’s . *On the , the elements are listed in order of their , which is the large whole number in each box. # of Protons = *In a neutral atom, the number of protons equals the number of . protons protons atomic number periodic table atomic number Atomic # electrons
Since the electron has an insignificant mass, the mass of an atom only depends on the number of and the atom has. protons neutrons Atomic Mass Protons + Neutrons =
change in # of Neutrons Isotopes = *Na-22 and Na-23 both have 11 protons and 11 electrons, but have 11 and 12 neutrons, respectively.
change in the # of Electrons Ions = *Na and Na+1 both have 11 protons, but have 11 and 10 electrons, respectively.