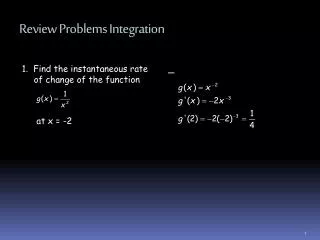
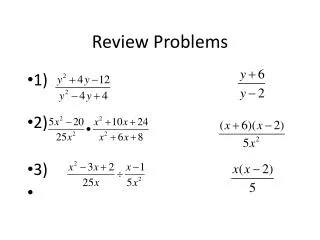
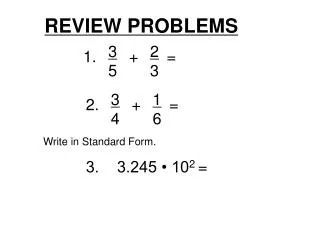
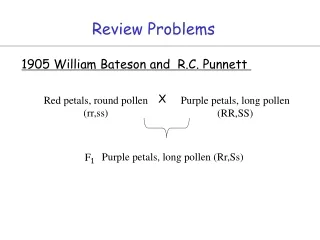
Review Problems
70 likes | 192 Vues
This report examines the reaction kinetics of water formation from hydrogen and oxygen gases in a sealed flask at 350 K. We analyze the rate of formation of H2O under different concentrations, resulting in calculated rates and deriving orders of reaction for both reactants. Using data from various concentrations, we deduce the average rate constant (k) and apply first-order kinetics to determine the time required to produce 0.01 moles of water, concluding it takes approximately 2.16 seconds.

Review Problems
E N D
Presentation Transcript
Water can be made from hydrogen and oxygen: H2 (g) + O2 (g) →H2O (g) Some experiments are run in a sealed 1 L flask at 350 K to determine the initial rate of reaction for different concentrations of reactants: [H2 ] M [O2] M Rate of formation of H2O M/sec 0.050 0.100 0.0048 0.050 0.050 0.0025 0.100 0.025 0.00120 If I began with 0.050 moles of both reactants, how long would it take to make 0.01 moles of water?
Orders of reaction [H2 ] M [O2] M Rate of formation of H2O M/sec 0.050 0.100 0.0048 0.050 0.050 0.0025 0.100 0.025 0.00120 Rate = k[H2]x[O2]y Rate 1 = k[0.050]x[0.100]y Rate 2 = k[0.050]x[0.050]y 0.0048 = [0.100]y 0.0025 = [0.050]y 1.92 =2y Y =1
Orders of reaction [H2 ] M [O2] M Rate of formation of H2O M/sec 0.050 0.100 0.0048 0.050 0.050 0.0025 0.100 0.025 0.00120 Rate = k[H2]x[O2]y Rate 2 = k[0.050]x[0.050] Rate 3 = k[0.100]x[0.025] 0.0025 = [0.100]x 2 0.0012 = [0.050]x 2.08 =2*2x 1.04 =2x X=0
Rate = k[O2] [H2 ] M [O2] M Rate of H2O M/sec 0.050 0.100 0.0048 0.050 0.050 0.0025 0.100 0.025 0.00120 0.0048 M/s = k [0.100 M] K =0.048 s-1 0.0025 = k [0.050] K =0.05 s-1 0.0012 =k[0.025] K =0.048 s-1 Kavg = 0.0487 s-1
1st order kinetics ln[O2]t= - kt [O2]0 2H2 (g) + O2 (g) →2H2O (g) If I began with 0.050 moles of both reactants, how long would it take to make 0.01 moles of water? 0.010 mol H2O* 1 mol O2 = 0.005 mol O2 2 mol H2O 0.050 mol O2 started – 0.005 mol O2 reacted = 0.045 mol left
1st order kinetics ln [O2]t= - kt [O2]0 Ln (0.045/0.05) = - 0.0487s-1 t t = 2.16 seconds!