### Unraveling the Atom: The Building Blocks of Matter ###
520 likes | 559 Vues
Delve into the atomic structure, from the indivisible atom to its nucleus and electron cloud. Learn about ions, isotopes, and the minuscule scale of particles within an atom. Discover the staggering difference in size between the nucleus and electron cloud. Explore the concept of atomic mass units and the historical journey of understanding the atom through indirect evidence. ###

### Unraveling the Atom: The Building Blocks of Matter ###
E N D
Presentation Transcript
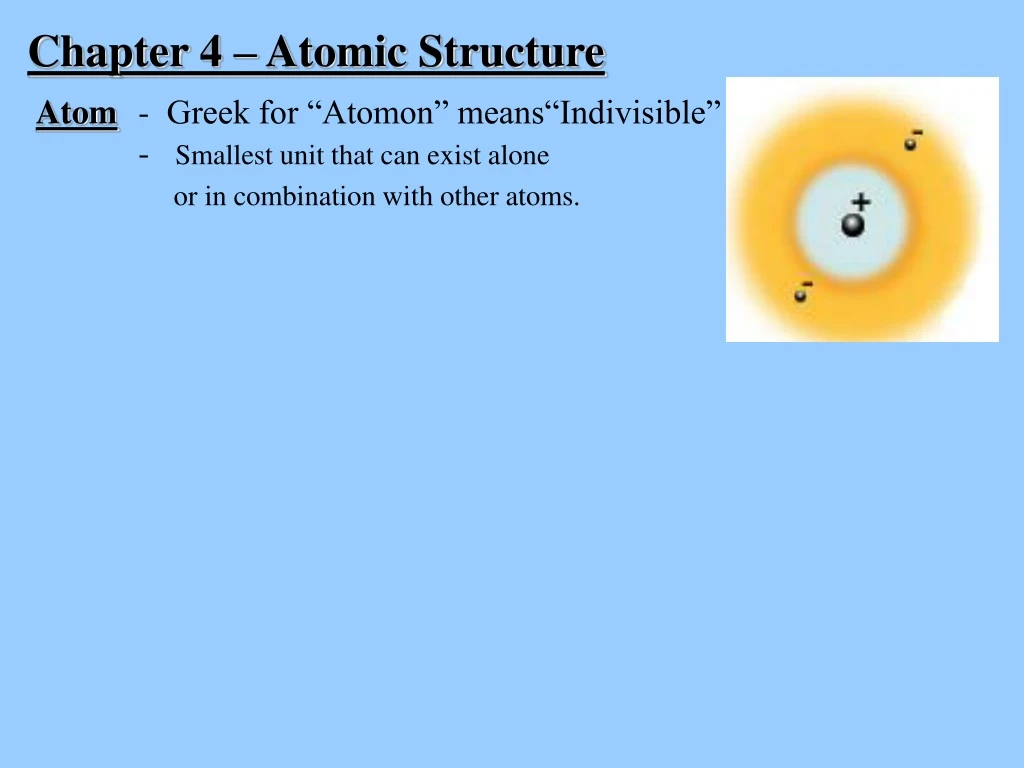
Chapter 4 – Atomic Structure Atom- Greek for “Atomon” means“Indivisible” - Smallest unit that can exist alone or in combination with other atoms.
Chapter 4 – Atomic Structure Atom- Greek for “Atomon” means“Indivisible” - Smallest unit that can exist alone or in combination with other atoms. Structure of an Atom … Things we know Two main areas of the atom: Nucleus Electron cloud
Chapter 4 – Atomic Structure Atom- Greek for “Atomon” means“Indivisible” - Smallest unit that can exist alone or in combination with other atoms. Structure of an Atom … Things we know Two main areas of the atom: Nucleus - contains protons (+) and neutrons (0) - dense, small Electron cloud - contains electrons (-) - surrounds nucleus, mostly empty space - arranged in shells or energy levels
Chapter 4 – Atomic Structure Atom- Greek for “Atomon” means“Indivisible” - Smallest unit that can exist alone or in combination with other atoms. Structure of an Atom … Things we know Two main areas of the atom: Nucleus - contains protons (+) and neutrons (0) - dense, small Electron cloud - contains electrons (-) - surrounds nucleus, mostly empty space - arranged in shells or energy levels Atom itself is neutral: Why? Number of p+ = e- n0 are neutral - no effect
Ions- atoms or molecules in which the total number of electrons does NOT equal the total number of protons. These particles have a CHARGE. Cations- positive ions (lose electrons) Anions- negative ions (gain electrons)
Isotopes – atoms of the same element with different masses due to a different number of n0 Ex. H has 3 isotopes 1 2 3 H H H 1 1 1 1 p+ 1 p+ 1 p+ 1 e- 1 e- 1 e- 1 e- 1 n0 2 n0 The only thing that changes is the neutrons… …so it is still hydrogen and acts like hydrogen but it is a little heavier with each additional neutron added…
More on Structure of an Atom … Things we know… Atom is very, very small overall→ we measure it in Angstroms 1 A = 1 x 10-10 m
More on Structure of an Atom … Things we know… Atom is very, very small overall→ we measure it in Angstroms 1 A = 1 x 10-10 m Compare size of electron cloud to nucleus Nucleus is extremely tiny compared to the electron cloud. How much smaller?????
More on Structure of an Atom … Things we know… Atom is very, very small overall→ we measure it in Angstroms 1 A = 1 x 10-10 m (compare to Metric system units) 10 mill A = 1 mm Compare size of electron cloud to nucleus Nucleus is extremely tiny compared to the electron cloud. Nucleus “marble” How much smaller????? Nucleus is a marble in the middle of a football stadium. Electron Cloud “football field”
More on Structure of an Atom … Things we know… Compare size of proton, neutron, and electron
More on Structure of an Atom … Things we know… Compare size of proton, neutron, and electron Nucleus Proton’s mass = 1 amu Neutron’s mass = 1 amu
More on Structure of an Atom … Things we know… Compare size of proton, neutron, and electron Electron’s mass = 0 amu Nucleus Proton’s mass = 1 amu Electron Cloud Neutron’s mass = 1 amu
More on Structure of an Atom … Things we know… Compare size of proton, neutron, and electron Interesting Stuff … all the mass of the atom is in the nucleus…Wow! What does that mean about the density of the nucleus? Electron’s mass = 0 amu Nucleus Proton’s mass = 1 amu Electron Cloud Neutron’s mass = 1 amu
More on Structure of an Atom … Things we know… Compare size of proton, neutron, and electron Interesting Stuff … all the mass of the atom is in the nucleus…Wow! What does that mean about the density of the nucleus? Electron’s mass = 0 amu Nucleus Proton’s mass = 1 amu Electron Cloud Neutron’s mass = 1 amu 1 amu (atomic mass unit) = 1.673 x 10-24 g The atomic mass unit is easier to use to describe something so ridiculously small! 0.000000000000000000000001673 g
Does an electron having “0” amu means it weighs nothing? Not Exactly … It’s just it is so small it does not matter relatively …
More on Structure of an Atom … Things we know… A word about the … amu *amu – atomic mass unit – special unit for the mass of an atom. 1/12 mass of carbon-12 atom. (relative to atom)
More on Structure of an Atom … Things we know… A word about the … amu *amu – atomic mass unit – special unit for the mass of an atom. 1/12 mass of carbon-12 atom. (relative to atom) Similar to: 2000 lbs. = 1 ton Easier to say 10 tons than 20,000 lbs. Just like: 1 amu (atomic mass unit) = 1.673 x 10-24 g Easier to say 2 amu than 2.346 x 10-24 g
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History Before I start into the history… …a little bit about Indirect Evidence??? Where to hunt? Measure thickness of paper or a dollar?
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History 400 BC Democritus The idea of the atom stems back to 400 BC by a Greek thinker named Democritus …he called matter “atomon” meaning “indivisible”
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History Now, this guy did not have any proof…he just thought about it and told people what he thought … 400 BC …Good Guess?? Democritus The idea of the atom stems back to 400 BC by this Greek thinker …he called matter “atomon” meaning “indivisible”
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... 1782 A Little Bit of History Antoine Lavoisier Law of conservation of mass matter cannot be created nor destroyed
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... H2O A Little Bit of History two H’s one O For this compound to be water… it must have exactly 2 H and 1 O What is this? H2O2 1799 Joseph Proust Law of definite proportions a chemical compound contains the same elements in exactly the same proportions by mass
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... Carbon monoxide CO C = 12 g O = 16 g Carbon dioxide CO2 C = 12 g O = 32 g A Little Bit of History Ratio 1 : 1 1803 Ratio 1 : 2 Same elements but different ratios make different compounds John Dalton Law of multiple proportions If compounds are composed of the same elements, the masses of the elements can be expressed as ratios of small whole #’s.
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History 1803 John Dalton The Atomic Theory • All matter is composed of extremely small, indivisible particles, called atoms. • Atoms of the same element are chemically alike. Atoms of different elements are chemically different. . • Atoms combine in whole # ratios to form compounds. • Atoms are combined, separated, or rearranged in chemical reactions.
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History Joseph Thompson Used a cathod ray tube to prove there were negative charged particles (now known as electrons) in an atom. This opened the way to the idea that an atom was not just a solid sphere not able to be broken down anymore. 1897
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History Earnest Rutherford 1911 In the gold foil experiment, he proved that the electron cloud was huge in volume comparison to the nucleus and the nucleus was extremely dense.
How did we figure out all this stuff about the atom??? Because theatomis so small !!!!! How about... A Little Bit of History Neils Bohr 1913 He proposed a model of the atom that showed that e- circled that nucleus of an atom in only allowed orbits or paths.
The Evolving Atomic Model Summary Dalton Model 1803 – John Dalton believed that an atom was an indestructible particle with no internal frame. (Billiard Ball Model) Thomson Model 1897 – J.J. Thomson discovers the electron. He believed electrons were embedded in positive charge sphere. (Plum pudding Model) Rutherford Model 1911 – Ernest Rutherford discovers that there is a dense, positively charged nucleus. Electrons go around the nucleus. Bohr Model 1913 – Niels Bohr enhances Rutherford’s model by having electrons move in a circular orbit at fixed distances from the nucleus.
Atomic Number – number of p+ in the nucleus of an atom (always equal to number of e-) Mass Number – number of p+ and n0 in the nucleus of an atom Atomic Weight (mass) – the average mass of the isotopes The mass number is just the atomic weight rounded off to a whole number!! Atomic Weight 32.065 Shorthand method: 32 S 16 Mass # (rounded) 32 Atomic # (# of p+ or e-)- Put light blue boxes on your periodic table…as a reference Mass # - Atomic # = n0
Atomic Weight = (Avg. mass of isotopes) = 32.066 Atomic # = (number of protons or electrons) = 16 Mass # = (Atomic weight rounded) = 32 # p+ = (same as Atomic #) = 16 p+ # e - = (same as Atomic #) = 16 e - # n0 = (Mass # - Atomic #) = 16 n0 (32 – 16) Try Sodium (Na): Atomic Weight = (Avg. mass of isotopes) = ________ Atomic # = (number of protons) = ________ Mass # = (Atomic weight rounded) = ________ # p+ = (same as Atomic #) = ________ # e - = (same as Atomic #) = ________ # n0 = (Mass # - Atomic #) = ________
Atomic Weight = (Avg. mass of isotopes) = 32.066 Atomic # = (number of protons or electrons) = 16 Mass # = (Atomic weight rounded) = 32 # p+ = (same as Atomic #) = 16 p+ # e - = (same as Atomic #) = 16 e - # n0 = (Mass # - Atomic #) = 16 n0 (32 – 16) Try Sodium (Na): Atomic Weight = (Avg. mass of isotopes) = ________ Atomic # = (number of protons) = ________ Mass # = (Atomic weight rounded) = ________ # p+ = (same as Atomic #) = ________ # e - = (same as Atomic #) = ________ # n0 = (Mass # - Atomic #) = ________ 22.99 11 23 11 11 12
PRACTICE THESE………… Au Ag Pb Atomic Weight = (Avg. mass of isotopes) = _______________ Atomic # = (number of protons) = ______________ Mass # = (Atomic weight rounded) = ______________ # p+ = (same as Atomic #) = ______________ # e - = (same as Atomic #) = ______________ # n0 = (Mass # - Atomic #) = ______________
PRACTICE THESE………… AuAgPb Atomic Weight = (Avg. mass of isotopes) = __________________ Atomic # = (number of protons) = __________________ Mass # = (Atomic weight rounded) = __________________ # p+ = (same as Atomic #) = __________________ # e - = (same as Atomic #) = __________________ # n0 = (Mass # - Atomic #) = __________________ 196.97 79 197 79 79 118 107.87 47 108 47 47 61 207.2 82 207 82 82 125
Shorthand Notation Mass # Atomic #
Ions- atoms or molecules in which the total number of electrons does NOT equal the total number of protons. These particles have a CHARGE. Cations- positive ions (lose electrons) Anions- negative ions (gain electrons)
Periodic Table • Horizontal Rows are periods. • Indicate how many shells are needed to hold all of the electrons • Vertical columns are Groups or families • Indicate the # of valence electrons 1 2 3 4 5 6 7 8
Ions • NonMetals • Gain electrons until they have 8* • Form negative ions • Equal to how many electrons they gained • Anions • Metals • Lose Valence electrons • Form Positive Ions • Equal to Group # • Cations 1 2 3 4 5 6 7 8 *Helium has 2 valence e- *Silver has 1 valence e- * *
Ions NonMetals (end in “ide”) F-1 Fluoride Metals (regular name) Ca+2 Calcium Ion 1 2 3 4 5 6 7 8
Isotopes – atoms of the same element with different masses due to a different number of n0 Ex. H has 3 isotopes 1 2 3 H H H 1 1 1 1 p+ 1 p+ 1 p+ 1 e- 1 e- 1 e- 1 e- 1 n0 2 n0 The only thing that changes is the neutrons… …so it is still hydrogen and acts like hydrogen but it is a little heavier with each additional neutron added…
If you change the number of neutrons…it is still Carbon but it makes a different isotope. Carbon –12 Carbon - 14 Different number of neutrons 8
Isotopes? Which of the following represent isotopes of the same element? Which element?
What is an Average Atomic Mass (weight)? Look at our periodic table…why the decimals???? It comes from the isotopes…Let’s look at Oxygen… 16 17 18 O O O 8 8 8 8 p+ 8 p+ 8 p+ 8 e- 8 e- 8 e- 1 e- 8 n0 9 n0 10 n0 .20% 0.038% 99.762% Almost all of Oxygen is Oxygen 16 but there are small amounts of the others…therefore it makes sense that the average atomic mass would be closest to 16…here is how it is figured … Oxygen 16 Oxygen 17 Oxygen 18 (15.994915)(0.99762) + (16.999131)(0.00038) + (17.999160)(0.0020) = 15.9994 average atomic mass
Isotope Calculations (abundance as a decimal) ( mass of isotope) + (abundance as a decimal) ( mass of isotope) + (abundance as a decimal) ( mass of isotope) Answer =
Isotope Calculations Element X has two natural isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has a relative abundance of 80.09%. 10.810 amu Answer =
Lewis Dot Structures • Find your element on the periodic table. • Determine the number of valence electrons. • This is how many electrons you will draw
Lewis Dot Structures • Write the element symbol. • Write down the number of valence electrons (based of the group #) • Write down the symbol and draw an imaginary box around it • Draw the valence electrons as dots on the sides of the box. • One at a time on each side until you are out of valence electrons N
Lewis Dot Structures On an open area on your notes, try these elements on your own: • H • P • Ca • Ar • Cl • Al
Bohr Model of the Atom – …Kinda like concert seating! Put the number of p+ , n0 , and e-in the diagram as shown… Lets diagram Sulfur p+ = Atomic # = 16 e- = Atomic # = 16 n0 = Mass # - Atomic # = 32 – 16 = 16 Remember that mass # is atomic weight rounded Maximum seats = 2 8 18 32 p+ = 16 e- = 2 e- = 2 e- = 8 e- = 2 e- = 6 e- = n0 = 16