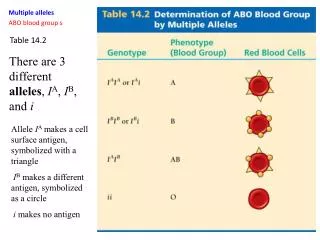
EXAMPLE 14.2
160 likes | 335 Vues
Solution : We must identify the acid and the base and know that they react to form water and a salt. Notice that Ca(OH) 2 contains 2 mol of OH – for every 1 mol of Ca(OH) 2 and will therefore require 2 mol of H + to neutralize it. We first write the skeletal reaction.

EXAMPLE 14.2
E N D
Presentation Transcript
Solution: We must identify the acid and the base and know that they react to form water and a salt. Notice that Ca(OH)2 contains 2 mol of OH– for every 1 mol of Ca(OH)2 and will therefore require 2 mol of H+ to neutralize it. We first write the skeletal reaction. We then balance the equation. FOR MORE PRACTICE Example 14.12; Problems 45, 46. SKILLBUILDER 14.2 Writing Equations for Neutralization Reactions Write a molecular equation for the reaction that occurs between aqueous H3PO4 and aqueous NaOH. Hint: H3PO4 is a triprotic acid, meaning that 1 mol of H3PO4 requires 3 mol of OH- to completely react with it. EXAMPLE 14.2 Writing Equations for Neutralization Reactions Write a molecular equation for the reaction between aqueous HCl and aqueous Ca(OH)2.
Given: 010.00 mL HCl solution 12.54 mL of a 0.100 M NaOH solution Find:concentration of HCl solution (mol/L) Equation: EXAMPLE 14.4 Acid–Base Titration The titration of 10.00 mL of an HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the endpoint. What is the concentration of the unknown HCl solution? You are given the volume of an unknown HCl solution and the volume of a known NaOH solution required to titrate the unknown solution. You are asked to find the concentration of the unknown solution. You will need two equations. The first is the equation for the neutralization reaction of HCl and NaOH (which you should write using your knowledge of acid–base reactions). The second is simply the definition of molarity
Solution Map: Solution: EXAMPLE 14.4 Acid–Base Titration Continued The solution map has two parts. In the first part, use the volume of NaOH required to reach the endpoint to calculate the number of moles of HCl in the solution. The final conversion factor comes from the balanced neutralization equation. In the second part, use the number of moles of HCl and the volume of HCl solution to determine the molarity of the HCl solution. Calculate the moles of HCl in the unknown solution by following the first part of the solution map. To get the concentration of the solution, divide the number of moles of HCl by the volume of the HCl solution in L. (Note that 10.00 mL is equivalent to 0.01000 L.) The unknown HCl solution therefore has a concentration of 0.125 M
SKILLBUILDER 14.4 Acid–Base Titration The titration of a 20.0-mL sample of an H2SO4 solution of unknown concentration requires 22.87 mL of a 0.158 M KOH solution to reach the endpoint. What is the concentration of the unknown H2SO4 solution? FOR MORE PRACTICE Example 14.14; Problems 53, 54, 55, 56, 57, 58. EXAMPLE 14.4 Acid–Base Titration Continued
FOR MORE PRACTICE Example 14.15; Problems 61, 62. SKILLBUILDER 14.5 Determining [H3O+] in Acid Solutions What is the H3O+ concentration in each of the following solutions? (a) 0.50 M HCHO2 (b) 1.25 M HI (c) 0.75 M HF EXAMPLE 14.5 Determining [H3O+] in Acid Solutions What is the H3O+ concentration in each of the following solutions? (a) 1.5 M HCl (b) 3.0 M HC2H3O2 (c) 2.5 M HNO3 Solution: (a) Since HCl is a strong acid, it completely ionizes. The concentration of H3O+ will be 1.5 M. [H3O+] = 1.5 M (b) Since HC2H3O2 is a weak acid, it partially ionizes. The calculation of the exact concentration of H3O+ is beyond the scope of this text, but we know that it will be less than 3.0 M. [H3O+] < 3.0 M (c) Since HNO3 is a strong acid, it completely ionizes. The concentration of H3O+ will be 2.5 M. [H3O+] = 2.5 M
FOR MORE PRACTICE Example 14.16; Problems 65, 66. SKILLBUILDER 14.6 Determining [OH–] in Base Solutions What is the OH– concentration in each of the following solutions? (a) 0.055 M Ba(OH)2 (b) 1.05 M C5H5N (c) 0.45 M NaOH EXAMPLE 14.6 Determining [OH–] in Base Solutions What is the OH– concentration in each of the following solutions? (a) 2.25 M KOH (b) 0.35 M CH3NH2 (c) 0.025 M Sr(OH)2 Solution: (a) Since KOH is a strong base, it completely dissociates into K+ and OH– in solution. The concentration of OH- will be 2.25 M. [OH–] = 2.25 M (b) Since CH3NH2 is a weak base, it only partially ionizes water. We cannot calculate the exact concentration of OH- but we know it will be less than 0.35 M. [OH–] < 0.35 M (c) Since Sr(OH)2 is a strong base, it completely dissociates into Sr2+(aq) and 2 OH-(aq). Sr(OH)2 forms 2 mol of OH- for every 1 mol of Sr(OH)2. Consequently, the concentration of OH- will be twice the concentration of Sr(OH)2. [OH–] = 2(0.025 M) = 0.050 M
SKILLBUILDER 14.8 Calculating pH from [H3O+] Calculate the pH of each of the following solutions and indicate whether the solution is acidic or basic. (a) [H3O+] = 9.5 × 10–9 M (b) [H3O+] = 6.1 × 10–3 M EXAMPLE 14.8 Calculating pH from [H3O+] Calculate the pH of each of the following solutions and indicate whether the solution is acidic or basic. (a) [H3O+] = 1.8 × 10–4 M (b) [H3O+] = 7.2 × 10–9 M Solution: To calculate pH, simply substitute the given [H3O+] into the pH equation. (a) pH = – log [H3O+] = – log 1.8 × 10–4 = – (– 3.74) = 3.74 Since the pH < 7, this solution is acidic. (b) pH = – log [H3O+] = – log(7.2 × 10–9) = – (– 8.14) = 8.14 Since the pH > 7, this solution is basic.
Solution: To find the [H3O+] from pH, we must undo the log function. Use either Method 1 or Method 2. EXAMPLE 14.9 Calculating [H3O+] from pH Calculate the H3O+ concentration for a solution with a pH of 4.80.
Solution: Skeletal equation: Balanced equation: EXAMPLE 14.12 Writing Equations for Neutralization Reactions Write a molecular equation for the reaction between aqueous HBr and aqueous Ca(OH)2
Given: 15.00-mL NaOH 17.88 mL of a 0.1053 M H2SO4 solution Find: concentration of NaOH solution (mol/L) Equation: Solution Map: EXAMPLE 14.14 Acid–Base Titrations A 15.00-mL sample of a NaOH solution of unknown concentration requires 17.88 mL of a 0.1053 M H2SO4 solution to reach the endpoint in a titration. What is the concentration of the NaOH solution?
Solution: The unknown NaOH solution has a concentration of 0.2510 M. EXAMPLE 14.14 Acid–Base Titrations Continued
EXAMPLE 14.15 Determining [H3O+] in Acid Solutions What is the H3O+ concentration in a 0.25 M HCl solution and in a 0.25 M HF solution? Solution: In the 0.25 M HCl solution (strong acid), [H3O+] = 0.25 M. In the 0.25 M HF solution (weak acid), [H3O+] < 0.25 M.
EXAMPLE 14.16 Determining [OH–] in Base Solutions What is the OH– concentration in a 0.25 M NaOH solution, in a 0.25 M Sr(OH)2 solution, and in a 0.25 M NH3 solution? Solution: In the 0.25 M NaOH solution (strong base), [OH–] = 0.25 M. In the 0.25 M Sr(OH)2 solution (strong base), [OH–] = 0.50 M. In the 0.25 M NH3 solution (weak base), [OH–] < 0.25 M.
Solution: EXAMPLE 14.17 Finding the Concentration of [H3O+] or [OH–] form Kw Calculate [OH–] in a solution with [H3O+] = 1.5 × 10–4 M.
EXAMPLE 14.18 Calculating pH from [H3O+] Calculate the pH of a solution with [H3O+] = 2.4 × 10–5 M. Solution: pH = – log[H3O+] = – log(2.4 × 10–5) = – (–4.62) = 4.62
EXAMPLE 14.19 Calculating [H3O+] frompH Calculate the [H3O+] concentration for a solution with a pH of 6.22. Solution: Method 1: Inverse Log Function [H3O+] = invlog(– pH) = invlog(– 6.22) = 6.0 × 10–7 Method 2: 10xFunction [H3O+] = 10–pH = 10–6.22 = 6.0 × 10–7