BRIDGING REACTION STEP 2
370 likes | 576 Vues
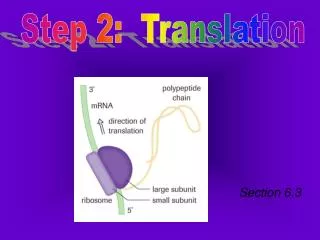
BRIDGING REACTION STEP 2. Fall 2013 BIOT 309. TRANSITION OR BRIDGING REACTION Connects glycolysis to citric acid/Kreb’s Cycle. OVERALL REACTION 2 pyruvate + 2 NAD + + 2 CoA-SH (coenzyme A) 2 acetyl-CoA + 2 NADH + 2 H + + 2 CO 2. CONNECTION TO OTHER BIOLOGY: Where else is CO 2 made?.

BRIDGING REACTION STEP 2
E N D
Presentation Transcript
BRIDGING REACTIONSTEP 2 Fall 2013 BIOT 309
TRANSITION OR BRIDGING REACTIONConnects glycolysis to citric acid/Kreb’s Cycle OVERALL REACTION 2 pyruvate + 2 NAD+ + 2 CoA-SH (coenzyme A) 2 acetyl-CoA + 2 NADH + 2 H+ + 2 CO2 CONNECTION TO OTHER BIOLOGY: Where else is CO2 made?
TRANSITION REACTION 3 carbon Co A 2 carbon
STEP 3 AEROBIC RESPIRATION:Krebs Cycle BIOT 309 Fall 2013
Tricarboxylic Acid Cycle = Krebs Cycle = Citric acid Cycle
THE TCA CYCLE • Converts acetyl CoA (from pyruvate via bridging reaction) to CO2 • Provides small amounts of energy in the form of GTP/ATP • Collects electrons and stores as NADH and FADH2 Electron Transport Chain (ETC) • Provides intermediates for other pathways • Occurs in cytoplasm
KREB’S CYCLE Summary Reaction: acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2H2O ——> 2CO2 + HSCoA + 3NADH + FADH2+ GTP + 2H+
CHEMICAL REACTIONS Key Equation: Δ G0 = -RTlnKeq • Δ G0 = Gibb’s standard free energy change, distance from equilibrium, (expresses driving force of reaction) • Keq =[products]/[reactants]; measurable • R= gas constant • T = absolute temperature (Kelvin)
BIOCHEMICAL REACTIONS • Instead of Δ G0, Δ G0’ is used • Δ G0’ =standard free energy change at pH 7.0 = biochemical standard free energy
Remember: enzymes, cofactors • Lower activation energy • Accelerate reaction • Organize and control reaction • Recover energy in new chemical forms and make it available for other uses
Gibb’s Free Energy • if Δ G0’ is negative, reaction goes forward spontaneously; - products have less energy than reactants • if Δ G0’ is ~ 0, reaction is at equilibrium • if Δ G0’ is positive, reaction does not go forward spontaneously • Δ G0’ of two or more reactions is calculated by adding reactions and the Δ G0’ of the reactions • CAVEAT: Δ G0 values shown in next slides will not be true under all circumstances, could be different for prokaryotes and eukaryotes
KREB’S CYCLE, step 1 2 C 4 C 6 C Citrate Synthase Aldol Condensation, X
KREB’S CYCLE, step 2 Aconitase Dehydration, Fe-S
KREB’S CYCLE, step 3 Aconitase Hydration, 4Fe-4S
Steps 2 & 3 combined STEPS 2 & 3 done by one enzyme aconitase Observe that: • Step 2: dehydration generates (double bond) intermediate (cis-aconitate) • Step 3: dehydration moves position of OH group
PRINCIPLE & EXAMPLE: Δ G0’ of overall reaction is calculated by adding reactions and the Δ G0’ of the reactions*: • Applied to 2 or more reactions, e.g., all of EMP or TCA citrate cis-aconitate Δ G0’ = +2 kcal/mol cis-aconitate isocitrate Δ G0’ = -0.5 kcal/mol citrate isocitrate Δ G0’ = +1.5 kcal/mol
KREB’S CYCLE, step 4 NADH, H+ NAD+ 6 C 5 C Isocitrate Dehydrogenase 2 step reaction Oxidative decarboxylation, Mg2+ or Mn2+ SPONTANEOUS
KREB’S CYCLE, step 5 NADH, H+ NAD+ +CoA-SH 5 C 4 C α-Ketoglutarate Dehydrogenase Complex Oxidative Decarboxylation, TPP, Lipoic Acid, FAD SPONTANEOUS
KREB’S CYCLE, step 6 GTP converted into ATP by nucleoside diphosphate kinase Succinyl CoA Synthetase Substrate Level Phosphorylation, FAD, TPP, Lipoic Acid
KREB’S CYCLE, step 7 • Why FAD? • alkane oxidation poorly exergonic and can’t reduce NAD+ Succinate Dehydrogenase Oxidation, FAD & FeS
KREB’S CYCLE, step 8 Fumarate Hydratase Hydration, Fe-S
KREB’S CYCLE, step 9 ΔG0’ = +7 kcal/mol Malate Dehydrogenase Oxidation
< > > *
KREB’S CYCLE !!! Summary Reaction: acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2H2O ——> 2CO2 + HSCoA + 3NADH + FADH2+ GTP + 2H+
Transition Reaction + Kreb’s Cycle Summary Reaction: 1 pyruvate + 4 NAD+ + 1 FAD + 1 GDP + 1 Pi ——> 4 CO2 + 4 NADH + 4 H+ + 1 FADH2+ 1 GTP(1 ATP)
EMP + TR + TCA Summary Reaction: GLUCOSE + 2H20 + 10 NAD+ 2 FAD + 4 ADP + 4 Pi ——> 6 CO2 + 10 NADH + 10 H+ + 4 ATP + 2FADH2
GLYOXYLATE CYCLEKREBS CYCLE ALTERNATIVE BIOT 309 Fall 2013
GLYOXYLATE SHUNT/CYCLE • By-passes 2 decarboxylation steps in TCA making possible • net formation of succinate, oxaloacetate, and other cycle intermediates from acetyl-CoA • Retains the two carbons lost in decarboxylation steps with each turn of TCA • => net synthesis of oxaloacetate, a four-carbon molecule, because each turn of the cycle incorporates two molecules of acetyl-CoA • Oxaloacetate used for other purposes
GLYOXYLATE SHUNT/CYCLE • Allows many bacteria to metabolize two-carbon substrates such as acetate FOR EXAMPLE: E. coli can be grown in a medium that provides acetate as the sole carbon source. E. coli synthesize acetyl-CoA, then uses it for energy production (via the citric acid cycle)
GLYOXYLATE SHUNT/CYCLE • Some enzymes in common with TCA • BUT has two exclusive enzymes not in TCA • isocitrate lyase: cleaves D-isocitrate to glyoxylate and succinate • malate synthase: forms L-malate from glyoxylate and acetyl-CoA
GLYOXYLATE SHUNT/CYCLE • Used when the principal or sole carbon source is a C2 compound (acetate, ethanol). • Fat catabolism produces acetyl CoA which feeds into other catabolic reactions and produces energy