Naming Covalent Compounds
200 likes | 686 Vues
Naming Covalent Compounds. Naming Covalent Compounds. You will use this naming techniques when you have a compound made of only two nonmetals The amount of each elements in covalent compounds are determined by charge (because they don’t have charges)

Naming Covalent Compounds
E N D
Presentation Transcript
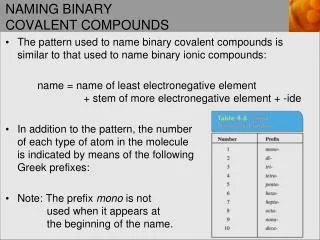
Naming Covalent Compounds • You will use this naming techniques when you have a compound made of only two nonmetals • The amount of each elements in covalent compounds are determined by charge (because they don’t have charges) • So you must indicate how many of each element you have with a prefix
Naming Covalent Compounds • 1 • 2 • 3 • 4 • 5 • 6 • 7 • 8 • 9 • 10 • mono (never used on 1st element) • di • tri • tetra • penta • hexa • hepta • octa • nona • deca
Naming Covalent Compounds • H2O • NH3 • CH4 • P2O5 • C3H8 • dihydrogen monoxide (water) • nitrogen trihydride (ammonia) • carbon tetrahydride (methane) • diphosphoruspentoxide • tricarbonoctahydride
Naming Covalent Compounds • carbon dioxide • carbon monoxide • sulfur trioxide • dinitrogenheptachloride • xenon hexafluoride • CO2 • CO • SO3 • N2Cl7 • XeF6
Naming Acids • All compounds that start with a hydrogen are acids (except water) • We will look at 2 cases • H bonded to halogen • H bonded to polyatomic ions
H-halogens • Naming • hydro___________ic acid • HCl • hydrochloric acid • HF • hydrofluoric acid
H-halogens • If you see a acid that starts with hydro it is a H bonded to halogen • hydroiodic acid • HI • hydrobromic acid • HBr
H-polyatomic ion • We will look at polyatomic ions that end with –ate or end with –ite • If the polyatomic ion ends in –ate change the ending to –ic acid • Ex: HNO3, NO3 is nitrate so HNO3 is nitric acid • If the polyatomic ion ends in –ite change the ending to –ous acid • Ex: HNO2, NO2 is nitrite so HNO3 is nitrous acid
H-polyatomic ion • H2CO3 • HBrO • HMnO4 • H2SO4 • H2SO3 • H3PO4 • H3PO3 • carbonic acid • hypobromous acid • permanganic acid • sulfuric acid • sulfurous acid • phosphoric acid • phosphorous acid
H-polyatomic ion • If you have an acid that doesn’t start with hydro and ends in –ic acid, the polyatomic ion ends in –ate or id it ends in –ouc acid, the polyatomic ion ends in –ite. • chloric acid • chlorous acid
H-polyatomic ion • silicic acid • arsenous acid • acetic acid • perbromic acid • hypofluorous acid • hyposulfurous acid • H2SiO3 • H3AsO3 • HC2H3O2 or CH3COOH • HBrO4 • HFO • HSO2