Mastering Covalent Compound Naming
70 likes | 115 Vues
Learn how to name covalent compounds efficiently with guidelines, prefixes, and examples provided. Understand the importance of using the same system for effective communication. Enhance your knowledge for interpreting food packaging and news updates.

Mastering Covalent Compound Naming
E N D
Presentation Transcript
Naming Covalent Compounds Chapter 19
Naming of Covalent Compounds • Why do we care? • Scientists must all use the same system so that we can communicate • Helps you to understand the news and packaging of foods
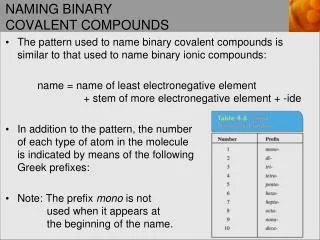
Naming Covalent Compounds • Name 1st element in the compound & add a prefix to indicate how many are present. • Name 2nd element by using the root of the element & adding the suffix –ide. • Then add a prefix to indicate how many are present. • When the name of the element begins with “a” or “o” and the prefix ends in “a” or “o”, drop the vowel at the end of the prefix. (For example monoxide, not monooxide.) • Diatomic elements are just named using the element name, • O2 is just oxygen
Diatomics • http://www.unis.org/UNIScienceNet/MOLEC_knowledge.html
Prefixes • Mono-1 • Di-2 • Tri-3 • Tetra- 4 • Penta- 5 • Hexa- 6 • Hepta- 7 • Octa- 8 • Nona- 9 • Deca- 10
Examples of Covalent Naming • SO3 • Sulfur Trioxide • CO • Carbon Monoxide • PCl5 • Phosphorous Pentachloride • SF6 • Sulfur hexafluoride
Writing Covalent Formulas • The prefixes tell you the subscript of each element within the formula • Dihydrogen monoxide: H2O