Membrane structure & function
170 likes | 318 Vues
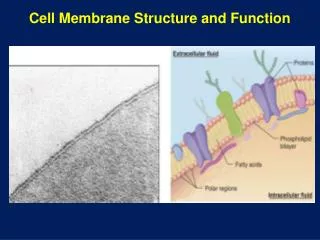
Membrane structure & function. Integral proteins. Can have any number of transmembrane segments Multiple transmembrane segments: often small molecule transport. Transport example: Bacteriorhodopsin. Proton pump: establish H + gradient Subsequent ATP generation

Membrane structure & function
E N D
Presentation Transcript
Integral proteins • Can have any number of transmembrane segments • Multiple transmembrane segments: often small molecule transport
Transport example: Bacteriorhodopsin • Proton pump: establish H+ gradient Subsequent ATP generation • 7 transmembrane segments of ~20 AA • a-helical • Hydrophobic interaction anchor • Pore for H+ movement • Interior of helices has some polar/charged character
1° structure: predict transmembrane segments“Hydropathy plots” • Predict whether sequence is Hydrophobic enough to cross membrane • Measure the DG when AA transferred from Hydrophobic into H2O • Calculate a ‘hydropathy index’ for a particular segment • If index of region > 0 → transmembrane segment
Tyr and Trp • Higher presence at membrane interface in integral proteins • Can interact both with lipids and H2O • Tyr (orange), Trp (red), charged (purple)
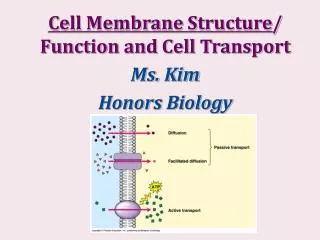
How do molecules cross the membrane? • Membrane fusion • uptake and release without “crossing a membrane” • Endocytosis: internalization of a vesicle • Exocytosis • Requires • Two bilayers recognize each other • Bilayers become closely ‘apposed’ • In position to fuse • Local disruption of bilayers • Fusion of bilayers to form a continuous surface • Mediated by fusion proteins • Recognition and local distortion
Simple diffusion: permeable divider (ie. solute able to diffuse through the membrane) • Uncharged species (polar or nonpolar) • Based on concentration gradient • Solute: net diffusion toward dilute side • At equilibrium: no net diffusion
Simple diffusion • Charged species • Concentration gradient and electrical gradient (membrane potential Vm) • Drives ions to reduce Vm • Ion movement depends on the electrochemical potential • Tend to equalize concentration AND equalize charge
Facilitated diffusion • Transporters or permeases that decrease Ea • Span lipid bilayer at least once • Movement only in thermodynamically favored direction • Affinity/specificity through weak forces • Classes • Carriers • Bind with high specificity • Saturable • Not very efficient • Monomers • Channels • Rapid transport • Less stereospecificity • Oligomers
Glucose transporter of erythrocytes • Required for metabolism • Transport of glucose from plasma into cells • Uniport system (one solute) • 50000 x faster transport • ~12 transmembrane regions (hydropathy plots) • a helices that have an polar/electrostatic channel for transport
Glucose transporter of erythrocytes • Behaves like a MM enzyme • Saturation effects • Model one glucose binds at a time • No covalent bonds • Fully reversible process • Concentration gradient dependent • Passive transport
Active transport • Solute accumulation against equilibrium • movement from low to high [solute] • Thermodynamically unfavorable: requires energy • 1° active transport • Coupled to exergonic chemical reaction • Commonly ATP hydrolysis • P-type, F-type, V-type, multidrug type • 2° active transport • Coupling of endergonic and exergonic transport of 2 different solutes • Exergonic process drives endergonic transport
Transport ATPases • P-type • Cation transporters • Reversible phosphorylation by ATP conf. change • V-type & F-type • H+ transport • Acidification of intracellular compartments (lysosomes) • Drives ATP synthesis • Multidrug transporters • Clinical significance • Transports drugs out of tumor cells or microbial cells • ‘multi-drug resistance’
P-type ATPases • Na+K+ ATPase • [Na+]intra low • [K+]intra high • Cells accumulate K+ and release Na+ • Control of cell volume, action potentials, sugar and AA transport • Each ATP hydrolyzed 3Na+ out and 2 K+ in • Membrane potential (Vm) -50-70 mV • Maintenance 20-40% metabolic energy of most cells
Na+K+ ATPase • Model • EnzI has high Na+ affinity • Enz II has high K+ affinity
Na+K+ ATPase Digitoxigenin-foxglove • Inhibitors • Ouabain • Digitoxigenin • Used as cardiac glycosides to treat congestive heart failure • Stabilize the E2-P complex • Na+ accumulation in cells • Antiporter (Ca2+ in and Na+ out) is activated • elevated cytosolic Ca2+ stimulates and strengthens contractions of heart muscles Strophanthus gratus