Understanding Thermochemistry: Energy Transfers, Specific Heat, and Reaction Enthalpy
190 likes | 337 Vues
Dive into the world of thermochemistry, where energy plays a vital role in chemical reactions and physical changes. Learn about the First Law of Thermodynamics, which states that energy cannot be created or destroyed, only transformed. Explore the differences between heat (measured in Joules) and temperature (Celsius or Kelvin). Understand specific heat and how different materials respond to heat energy, affecting temperature changes. Discover how exothermic and endothermic reactions release or absorb energy, defined by changes in enthalpy (ΔH). Finally, examine the heat of formation and combustion, shedding light on stability in chemical compounds.

Understanding Thermochemistry: Energy Transfers, Specific Heat, and Reaction Enthalpy
E N D
Presentation Transcript
17-1 Thermochemistry Transfers of energy as heat in chemical reactions and physical changes
Remember… The First Law of Thermodynamics: energy cannot be created or destroyed only converted from one form to another
Heat vs.Temperature • Heat (Joule, J) • measure of energy change in a system. • Temperature (Celsius, °C or Kelvin, K) • measure of the kinetic energy (movement) of the particles in a system. • Gaining or losing heat energy in a substance can change its temperature. • Exothermic • System loses energy to surroundings • Endothermic • System gains energy from surroundings
Specific Heat • is a property of matter, and different species have different Specific Heat. • The heat energy required to raise one gram of a pure substance 1° C or 1 K • The symbol we use is cp
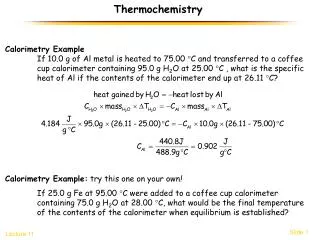
Specific Heat • Metals have very low cp, • which is why metals often feel cold to the touch. • Table on page 513 • Water has a very highcp, • 4.184 J/g·0C • Substances with lower cp will rise in temperature faster and require less energy to do so than do substances with high cp.
Specific Heat 1 calorie= 4.184 Joules
Specific Heat Equation cp = q/(m ΔT) OR q = cp x m x ΔT
Heat of Reaction • Quantity of energy released or absorbed during a chemical reaction • Thermochemical equation – shows the quantity of heat • Example: 2H2 + O2 2H2O + 483.6 kJ • Energy released or absorbed?
Heat of Reaction • Example: 2H2 + O2 2H2O + 483.6 kJ • Energy released (on product side) • EXOTHERMIC • What about when it’s on the reactant side?
Heat of Reaction • Example: 2H2O + 483.6 kJ 2H2 + O2 • Energy absorbed (on reactant side) • ENDOTHERMIC
Enthalpy Change (ΔH) • The amount of energy absorbed or lost ΔH = Hproducts - Hreactants • Thermochemical equations usually written this way 2H2 + O2 2H2O ΔH = -483.6 kJ/mol • When ΔH is negative, the system loses energy and it is EXOTHERMIC
Enthalpy Change (ΔH) • The amount of energy absorbed or lost ΔH = Hproducts - Hreactants 2H2O 2H2 + O2 ΔH = +483.6 kJ/mol • When ΔH is positive, the system gains energy and it is ENDOTHERMIC
Endothermic or Exothermic? C6H12O6 + 6O26CO2 + 6H20 ΔHrxn = -2870 kJ/mol
Heat of Formation (ΔHf) • energy released or absorbed to form 1 mole of a compound from its elements • p. 902 • If a large amount of energy is released when compound is formed… • Endothermic or exothermic? • Positive or Negative ΔHf?
Heat of Formation (ΔHf) • energy released or absorbed to form 1 mole of a compound from its elements • p. 902 • If a large amount of energy is released when compound is formed… • Endothermic or exothermic? • Positive or NegativeΔHf? • HIGH NEGATIVE ΔHf= VERY STABLE!
Heat of Formation (ΔHf) • POSITIVE ΔHf= UNSTABLE! • Pure elements ΔHf= O • ΔHfof carbon dioxide = -393.5 kJ/mol • More stable than C and O alone • HgC2N2O2ΔHf= +226.7 kJ/mol • Very unstable, used in explosives
Heat of Combustion (ΔHc) • Reactants? • Products? • Exothermic or Endothermic? • Positive or Negative?
Heat of Combustion (ΔHc) • Reactants? C and H with O2 • Products? CO2 and H2O and heat and light • Exothermic or Endothermic? • Positive or Negative? • P. 896
Stability of these compounds? • Al2O3 (s) -1676.0 kJ/mol • CaCO3 (s) -1206.92 kJ/mol • NO (g) 90.29 kJ/mol • O3 142.7 kJ/mol