Binary Ionic Compounds
120 likes | 571 Vues
Binary Ionic Compounds. Binary ionic compounds are made up of only two elements. The positive ion, or cation, is an ion consisting of only one atom. The name of this ion is the same as the name of the element. e.g. K + is a potassium ion; Mg 2+ is a magnesium ion.

Binary Ionic Compounds
E N D
Presentation Transcript
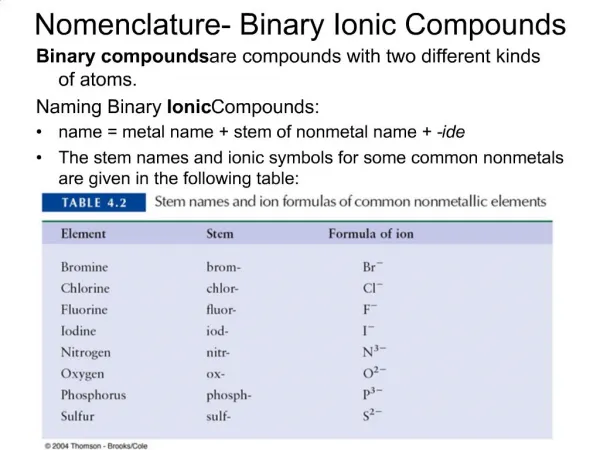
Binary ionic compounds are made up of only two elements. • The positive ion, or cation, is an ion consisting of only one atom. The name of this ion is the same as the name of the element. • e.g. K+ is a potassium ion; Mg2+ is a magnesium ion. • The negative ion, or anion, is an ion consisting of only one atom. The name of this ion is formed by changing the ending of the element to –ide. • e.g. F- is a fluoride ion; O2- is an oxide ion.
For atoms in the s and p blocks, the charges of the ions may be determined from their position in the periodic table.
Transition elements can sometimes form cations with more than one charge. • These cations are named by including their charge as a Roman numeral in parentheses. • e.g. Cu+ is the Copper (I) ion; • Cu2+ is the Copper (II) ion.
Cations and anions must be combined such that the total positive charges equal the total negative charges. • Example: Potassium bromide • K is found in the first column. Its charge is therefore +1. • Bromine is found in Group 17. Bromide’s charge is therefore -1. • To produce a neutral compound, one K+ is needed for every one Br-. • Therefore the formula is KBr.
Cations and anions must be combined such that the total positive charges equal the total negative charges. • Example: Magnesium chloride • Mg is found in the second column. Its charge is therefore +2. • Chlorine is found in Group 17. Chloride’s charge is therefore -1. • To produce a neutral compound, one Mg2+ is needed for every two Cl-. • Therefore the formula is MgCl2.
Cations and anions must be combined such that the total positive charges equal the total negative charges. • Example: Aluminum oxide • Al is found in the Group 13. Its charge is therefore +3. • Oxygen is found in Group 16. Oxide’s charge is therefore -2. • To produce a neutral compound, two Al3+ are needed for every three O2-. • Therefore the formula is Al2O3.
Transition elements can often have more than one charge. This charge will be the same as the Roman numeral. • Example: Iron (II) chloride • The iron ion has a charge of +2. • Chlorine is found in Group 17. Chloride’s charge is therefore -1. • To produce a neutral compound, one Fe2+ is needed for every two Cl-. • Therefore the formula is FeCl2.
Naming binary compounds • Name the ion with the cation first , the anion second. Examples: • NaCl: Sodium chloride • MgCl2: Magnesium chloride • CuCl2: Copper (II) chloride • Fe2O3: Iron (III) oxide • Note that the first word may or may not be capitalized, but the second word (the name of the anion) is never capitalized.
For atoms that form more than one ion, the Roman numeral indicating the charge must appear as part of the name. • Example: CuCl2 • Cu can have more than one charge. Assume that this is true for any transition element. • Chlorine is in group 17, so the chloride ion must have a charge of -1. • Since there are two Cl- for every one copper ion, the copper ion must have a charge of +2. • Therefore, the name is Copper (II) chloride.
For atoms that form more than one ion, the Roman numeral indicating the charge must appear as part of the name. • Example: Fe2O3 • Fe can have more than one charge. Assume that this is true for any transition element. • Oxygen is in group 16, so the oxide ion must have a charge of -2. • Since there are three 0-2 for every two iron ions, the iron ions must have a charge of +3. • Therefore, the name is Iron (III) oxide.