SOLUTIONS TO EXAMPLES
210 likes | 732 Vues
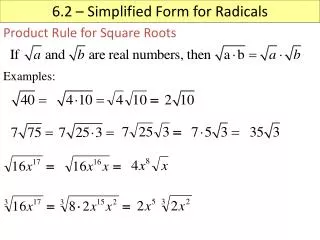
SOLUTIONS TO EXAMPLES. AgCl(s) Ag + (aq) + Cl - (aq) calculate [ Ag+] or [Cl - ]. Example 0. Solubility of AgCl(s) in water at 25 o C is 1.274 x 10 -5 mol kg -1 . Calculate the solubility of AgCl(s) in 0.010 mol kg -1 Na 2 SO 4 (aq).

SOLUTIONS TO EXAMPLES
E N D
Presentation Transcript
AgCl(s) Ag+(aq) + Cl-(aq) calculate [Ag+] or [Cl-] Example 0 Solubility of AgCl(s) in water at 25oC is 1.274 x 10-5 mol kg-1. Calculate the solubility of AgCl(s) in 0.010 mol kg-1 Na2SO4(aq). In the presence of Na2SO4 the solution is no longer ideal calculate activity coeff’s I < 0.05 Use Debye-Hückel law Calculate Ksp for the ideal soln and assume it be the same for the non-ideal soln Ksp = aAg+ aCl- For AgCl dissolving in H2O assume = 1 since m 0 Ksp = (1.27410-5)2 = 1.62310-10 For I = 0.030 mol kg-1 Ignore Ag+ and Cl- in solution as conc’s v. low Ksp = aAg+ aCl- = mAg+ mCl- 1.62310-10 = (0.666)2 m2 m = 1.9110-5 mol kg-1= solubility
Example 1 Consider the Daniell cell: Using the standard reduction potentials, calculate the equilibrium constant at 25 C. Standard reduction potentials at 25 C: Cu2+ + 2e- Cu(s) Eo = +0.34 V Zn2+ + 2e- Zn(s) Eo = −0.76 V Reduction rxn Oxidation rxn Overall reaction: Cu2+(aq) + Zn(s) Cu(s) + Zn2+(aq) The cell potential at standard conditions: E°cell > 0 cell reaction is spontaneous expect K > 1 = +1.10 V At equilibrium: Ecell = 0 V Cu2+(aq) + Zn(s) Cu(s) + Zn2+(aq) Since K is large reaction goes to completion K = 1.6 1037 Units: w = V q But J = C V
Example 2 The mean activity coefficients of HBr in 5.0 and 20.0 mmol kg–1 are 0.930 and 0.879, respectively. Consider a hydrogen electrode in HBr(aq) solution at 25 °C operating at 1.15 atm. Calculate the change in the electrode potential when the molality of the acid solution is changed from 5.0 and 20.0 mmol kg–1. Electrochemical reaction involved: 2H+(aq) + 2e H2(g) Let E1 and E2 be potentials for the initial and finalstates, i.e. for 5 and 20 mmol kg–1 and
Example 3 Devise a cell in which the cell reaction is: Mn(s) + Cl2(g) MnCl2(aq) Give the half reactions at the electrodes and from the standard cell potential of 2.54 V deduce the standard potential for the Mn2+/Mn(s) redox couple. Given: E°(Cl2/Cl-) = +1.36 V Ecell > 0 Galvanic cell spontaneous reaction written as above Half reactions: Reduction: Cl2(g) + 2e− 2Cl–(aq) E° = +1.36 V Suggest a Pt electrode as cathode. Oxidation: Mn(s)Mn2+(aq) + 2e− Mn2+(aq) + 2e− Mn(s) E° = ? V Suggest a Mn electrode as anode. Cell: Mn(s) | Mn2+(aq), Cl–(aq) | Cl2(g) | Pt(s) Standard potential for the Mn2+/Mn(s) redox couple: E°cell = E°cathode– E°anode = 1.36 V – x = 2.54 V E°anode = -1.18 V or E° for the redox couple Mn2+ / Mn(s) = -1.18 V
Example 4 Estimate the cell potential at 25°C for Ag(s)|AgBr(s)|KBr(aq, 0.050 mol kg–1)||Cd(NO3)2(aq,0.0034 mol kg–1)|Cd(s) E°(R-H) = –0.40 V E°(L-H) = +0.07 V (assume non-ideal solutions) Write the spontaneous electrochemical reaction. (R-H): Cd2+(aq) + 2e Cd(s) E° = –0.40 V (L-H): 2AgBr(s) + 2e 2Ag(s) + 2Br–(aq)E° = +0.07 V Cell reaction: Cd2+(aq) + 2Ag(s) + 2Br–(aq) Cd(s) + 2AgBr(s) E°cell = E°cathode – E°anode = E°R-H– E°L-H = – 0.47 V Need to calculate activities: ICd(NO3)2 = 0.010 and IKBr = 0.050 hence we can use the Debye-Hückel limiting law
-0.47 -0.084 -0.076 Ecell = –0.63 V = -0.076 = -0.084 Ecell < 0 non-spontaneous electrochemical reaction Cd2+(aq) + 2Ag(s) + 2Br–(aq) Cd(s) + 2AgBr(s) The spontaneous electrochemical reaction: Cd(s) + 2AgBr(s) Cd2+(aq) + 2Ag(s) + 2Br–(aq)
Example 5 The standard potential of the cell below at 25 °C is 0.95 V. Ag(s) |AgI(s) | AgI(aq) | Ag(s) Calculate: a) its solubility constantand b) the solubility of AgI. Note: the cell is considered at standard conditions and Ecell > 0 (R-H) Ag+(aq) + e- Ag(s) E° = +0.80 V (L-H) AgI(s) + e- Ag(s) + I–(aq)E° = –0.15 V Spontaneous electrochemical reaction: Ag+(aq) + I–(aq) AgI(s) E°cell = +0.95 V K = 1.11016 AgI(s) Ag+(aq) + I–(aq) Therefore Ksp = K–1 = 8.710–17 Ksp = [Ag+] [I-] = [Ag+]2 = 8.7 10–17 Solubility = [AgI(aq)] = 9.410–9 mol kg–1
Example 6 Calculate the degree of ionization and the acid dissociation constant at 298 K for a 0.010 M acetic acid solution that has a resistance of 2220 . The resistance of a 0.100 M potassium chloride solution was also found to be 28.44 . m(0.1 M KCl) = 129 Scm2 mol-1 o(H+) = 349.6 Scm2 mol-1 o(CH3COO-) = 40.9 Scm2 mol-1 Degree of ionisation, : where To find the cell constant (C), we can use the data for the KCl solution.
Degree of ionisation, : Using KCl data to find the cell constant (C): c = 0.100 M = 0.100 mol dm-3 = 1.0010-4 mol cm-3 Finding m: or 4.23%
Acid dissociation constant, Ka: CH3COOH+ H2O CH3COO-+ H3O+ Also pKa = -log(1.8710-5) = 4.73
Subtract two equations, solve for K. Note: is the same in for both eqn’s Example 7 The molar conductivity of a strong electrolyte in water at 25 °C was found to be 109.9 S cm2 mol-1 for a concentration of 6.2 10-3 mol L-1 and 106.1 S cm2 mol-1 for a concentration of 1.5 10-3 mol L-1. Estimate the limiting molar conductivity of the electrolyte. Note: Strong electrolyte Kohlrausch law 2 equations, 2 unknowns! = -0.095 S cm-2 Using K, calculate limiting molar conductivity from Kohlrausch law.
Example 8 The limiting molar conductivities of KCl, KNO3, and AgNO3 are 149,9, 145.0, and 133.4 S cm2 mol-1, respectively (all at 25 °C). What is the limiting molar conductivity of AgCl at this temperature? We can apply the Kohlrausch law of independent migration of ions. To solve: manipulate the 3 equations above to obtain the one for AgCl Recall:
Example 9 The mobility of the NO3- ion in aqueous solution at 25 °C is 7.4010-8 m2 s-1 V-1. (Viscosity of water is 0.89110-3 kg m-1 s-1). Calculate its diffusion coefficient and the effective radius at this temperature. Use the Einstein relation between the mobility and diffusion coefficient: Calculate the hydrodynamic radius: Or use the equation: Having “a” and the radius of a simple ion (without coordinated water) plus the dimension of a single water molecule, you would be able to predict the number of molecules in the hydrated share of the ion. Remember: in the calculations you have to show the work on units. Without that, the work might be considered as not done at all. J = kg m2 s-2 J = V C
Considering units: BUT w = qV J = C V BUT J = kg m2 s-2 Also: V = IR V = A and q = It C = As
Example 10 The electron transfer coefficient of a certain electrode in contact with the redox couple M3+ / M4+ in aqueous solution at 25 °C is 0.39. The current density is found to be 55.0 mA cm–2 when the overvoltage is 125 mV. What is the overvoltage required for a current density 75.0 mA cm–2? What is the exchange current density? Note that we deal with large and positive overpotential. Hence the Tafel equation for anodic current can be applied. M3+ M4+ + e- = 0.138 V j0 = 2.82 mA cm–2 j1 = 55.0 mA cm–2 1 = 0.125 V j2 = 75.0 mA cm–2 2 = ? F = 96485 C mol-1 R = 8.315 J K-1 mol-1 T = 298 Kn = 1 = 0.39
Example 11 The exchange current density and the electron transfer coefficient for the reaction 2H+ + 2e H2(g) on nickel at 25 °C are 6.3 10–6 A cm–2 and 0.58, respectively. Determine what current density would be required to obtain an overpotential of 0.20 V as calculated from the Butler-Volmer equation and the Tafel equation. Butler-Volmer: for a 2 electron process Tafel: The relatively high positive overpotential applied results in very little reduction taking place. Positive potential Anodic current F = 96485 C mol-1 R = 8.315 J K-1 mol-1T = 298 Kn = 2 = 0.58 = 0.20 V Jo = 6.3 10–6 A cm–2