Quantum model of the atom
140 likes | 365 Vues
Quantum model of the atom. http://youtu.be/45KGS1Ro-sc. Bohr’s theory was limited in it ability to predict line spectra of atoms other than hydrogen. It also could not account for many details of atomic structure of larger atoms.

Quantum model of the atom
E N D
Presentation Transcript
http://youtu.be/45KGS1Ro-sc • Bohr’s theory was limited in it ability to predict line spectra of atoms other than hydrogen. • It also could not account for many details of atomic structure of larger atoms
Today’s atomic theory is based on the principles of wave mechanics.
According to wave mechanics, electrons do not move in a definite path like planets orbiting the Sun.
In fact it is impossible to pin point the exact location of an electron. The PROBABLE location of an electron is described by its energy.
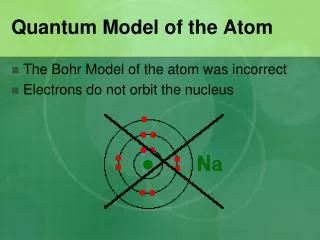
According to the new model an atom has a positive nucleus (with protons and neutrons) surrounded by a vast spacein which electrons are found. The space is called and electron cloud.
Depending on their energy electrons are locked into certain regions in the electron cloud. These regions are called energy levels. Electrons possessing the most energy are found furthest from the nucleus.
Within an energy level there are regions of regions of highest probability (or sub levels) called ORBITALS.
Each “shell” from the Bohr model is now seen as an energy level with ‘sub-levels’. A given energy level has a certain number of sub levels.
1st energy level (n = 1) has only an ‘s’ orbital (designated 1s) • 2nd energy level (n=2) has two orbitals ‘s’ and ‘p’ (designated 2s and 2p) • 3rd energy level (n=3) has three orbitals ‘s’, ‘p’ and d’. (designated 3s,3p and 3d) • 4th energy level has 4 orbitals.
Each sub level can accommodate a certain number of electrons: • s – 2 electrons • p – 6 electrons (there are three with 2 e- each) • d – 10 electrons (there are five with 2 e- each) • f – 14 electrons.
Orbital shapeshttp://youtu.be/K-jNgq16jEY • <iframe width="560" height="315" src="http://www.youtube.com/embed/K-jNgq16jEY" frameborder="0" allowfullscreen></iframe>