Classifying Matter
150 likes | 280 Vues
This text explores the classification of matter into elements, compounds, and mixtures. An element is defined as the simplest pure substance found on the Periodic Table, while a compound consists of two or more elements chemically combined. Mixtures, on the other hand, are physically combined substances that can be heterogeneous or homogeneous. Key concepts include solutions, the conservation of mass, and the distinction between various types of mixtures. Engage with hands-on experiments and discover the properties of unique mixtures like oobleck, showcasing non-Newtonian fluids.

Classifying Matter
E N D
Presentation Transcript
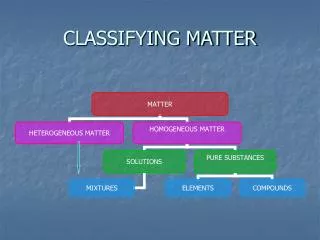
Elements An element is… The simplest pure substance One kind of material Definite properties Found on the Periodic Table of Elements
Compounds and Molecules A compound is… A pure substance One kind of material Definite properties Two or more elements chemically combined A molecule is the smallest particle of a compound that has all the properties of that compound Water is a compound H2O is a water molecule
Mixtures A mixture is… Matter Two or more substances Physically combined NOT chemically combined
Types of Mixtures Heterogeneous Mixtures Does not appear the same throughout Least Mixed Particles are large and easily separated Homogeneous Mixtures Appears to be the same throughout Well mixed Particles are small and not easily recognized
Types of Mixtures (cont) Heterogeneous Mixtures Homogeneous Mixtures
Solutions A solution is… A mixture Homogeneous Two or more substances A single physical state
Solutions (cont) Solvent – That part of a solution which doing the dissolving Solute – That part of a solution which is dissolved
Solutions (cont) Law of Conservation of Mass Atoms cannot be created nor destroyed Mass cannot be created nor destroyed Change results in arrangement of elements Mass will remain constant H = 1.0001 amu H2O = 17.9992 amu H = 1.0001 amu O = 15.999 amu
Testing Solution Chemistry (cont) Questions • Did your calculated (predicted) results equal your measured results? • Can you explain any differences? • In which scenarios were you able to get the salt to dissolve and in which did you have salt remaining at the bottom of the cup? • Did the amount of salt dissolved have any affect on your Conservation of Mass results? Use the following data about a 2-L bottle of Mountain Dew to answer Questions 5 through 8. Mass of Bottle = 55.6 Mass of Bottle + Soda = 2194 g Volume of Soda = 2000 ml Mass of 2000 ml of Soda = 2138.4 g Mass of Sodium = 360 mg Mass of Sugar = 276 g • The density of water = 1 g/ml. How much should 591 ml of water weigh? • How much (in grams) “stuff” is dissolved in the water to make it soda? • How much should a bottle of soda weigh? • Can you explain your observations and calculations?
Alloys An alloy is… A mixture A solution Two metals or A metal and a nonmetal
Suspensions A suspension is… A mixture Heterogeneous NOT a solution Two or more substances Particles large enough to settle and form a sediment
Ingredients and Procedure 2 parts corn starch 1 part water 1 – 2 drops of food coloring • Put corn starch in the cup • Add the food coloring • Slowly add water a few drops at a time and stir
Now for the science… Our cornstarch goo (sometimes referred to as “oobleck” from the Dr. Suess book) is what scientists call a “Non-Newtonian” liquid. Basically, Sir Issac Newton stated individual liquids flow at consistent, predictable rates. As you likely discovered, cornstarch goo does NOT follow those rules – it can act almost like a solid, and them flow like a liquid. Technically speaking, the goo is a SUSPENSION, meaning that the grains of starch are not dissolved, they are just suspended and spread out in the water. If you let the goo sit for an while, the cornstarch would settle to the bottom of the bowl. So why does this concoction act the way it does? Most of it has to do with pressure. The size, shape, and makeup of the cornstarch grains causes the cornstarch to “lock-up” and hold its shape when pressure is applied to it. People have filled small pools with oobleck and they are able to walk across the surface of it (as long as they move quickly.) As soon as they stop walking, they begin to sink.