Bonding
150 likes | 283 Vues
Bonding. Notes, packet p. 8-12 Review, packet p.16-17 . Metals vs. Nonmetals. Look for the ZIG-ZAG LEFT = Metals RIGHT = Nonmetals. Dot diagrams Determine the Dot Diagram for the following elements on p 8 in your packet. Be. C. Na. B. He. Dot diagrams. Be. C. Na. B. He.

Bonding
E N D
Presentation Transcript
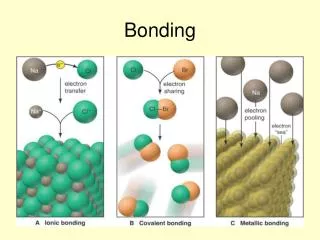
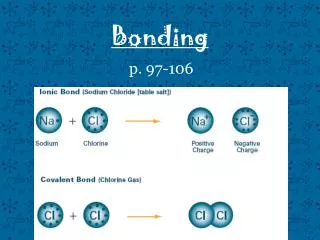
Bonding Notes, packet p. 8-12 Review, packet p.16-17
Metals vs. Nonmetals Look for the ZIG-ZAG LEFT = Metals RIGHT = Nonmetals
Dot diagramsDetermine the Dot Diagram for the following elements on p 8 in your packet. Be C Na B He
Dot diagrams Be C Na B He
If you change protons, you change the element.If you change the neutrons, you change the isotope.If you change the electrons, you change theion. New word for today!! An ION is a CHARGED ATOM (either + or - ) This happens when an unstable atom loses or gains electrons in order to become stable. (Remember: 8 valence electrons = HAPPY ATOM!
Neutral atoms have an equal number of protons and electrons. A neutral atom has an overall electrical charge of zero. BUT, a NEUTRAL atom isn’t necessarily a STABLE atom! Why would atoms gain or lose electrons? HAPPY ATOMS are stable meaning they fulfill the ‘Octet Rule’ (8 electrons in outer level). Metals will LOSE electrons (+ ion) = CATION Nonmetals will GAIN electrons (- ion) = ANION
Determining charge of an Ion Gain electron = NEGATIVE Lose electron = POSITIVE
Gain electron = NEGATIVE Lose electron = POSITIVE Determining charge of an Ion Answer the following questions on p. 9 in your packet.
Gain electron = NEGATIVE Lose electron = POSITIVE Determining charge of an Ion Answer the following questions on p. 9 in your packet. 8 electrons + 2 electrons = 10 electrons Now Oxygen has 2 MORE negative charges than positive, so charge is -2. 4 electrons - 2 electrons = 2 electrons Now Beryllium has 2 LESS negative charges than positive, so charge is +2. 16 + charges 18 - charges 2 more – than + -2 charge
Ion notation +1 +1 = 1 electron lost +2 = 2 electrons lost +3 = 3 electrons lost +4 = 4 electrons lost -1 = 1 electron gained -2 = 2 electrons gained -3 = 3 electrons gained -4 = 4 electrons gained Lose (+) or Gain (-) How many electrons
+4 or -4 Add to your periodic Table… +1 +2 +3 -1 -3 -2
Practice these on p. 9 in packet Remember… – is gain and + is lose!
Why haven’t you grown up recognizing all of the elements around you? Because most substances are compounds. Very few elements are stable enough to exist pure. Instead, they react with other elements to form compounds. Why?.... Atoms will gain, lose, or share electrons until their outer energy level contains 8 electrons. HAPPY ATOMS! THE OCTET RULE
Watch the BrainPOP video. Username: onlineschool Password: success Do a SEARCH for Ions Take the QUIZ!
Complete ‘Determining Ions’ worksheet using your notes and Periodic table. An example has been done for you! Ask questions if you have any.