The Periodic Table & Its Properties Chapters 5 & 6
1.12k likes | 1.37k Vues
The Periodic Table & Its Properties Chapters 5 & 6 . Chemistry-CP Periods 1, 2, 5 & 7. J.W. Dobereiner. In 1860, there were only 63 elements known Classified some elements into triads--groups of three

The Periodic Table & Its Properties Chapters 5 & 6
E N D
Presentation Transcript
The Periodic Table & Its PropertiesChapters 5 & 6 Chemistry-CP Periods 1, 2, 5 & 7
J.W. Dobereiner • In 1860, there were only 63 elements known • Classified some elements into triads--groups of three • Triads had: Similar chemical properties & physical properties that varied in an orderly way • Important because: He grouped elements with similar properties revealing an orderly pattern in the elements’ properties.
Examples of Triads • Halogen Triad: Chlorine, Bromine and Iodine • Coinage Triad: Copper, Silver & Gold • Metal Triad: Calcium, Strontium & Barium
J.A.R. Newlands (1865) • Realized that when the elements were arranged by increasing atomic mass, the properties of the 8th element were similar to the 1st element. • Law of Octaves: The periodic pattern repeats itself every 8 elements
Dmitri Mendeleev • Russian chemist who developed the first periodic table • Listed the elements according to atomic mass • Important because: He showed the properties of the elements repeat in an orderly way from row to row of the table
Periodicity: the tendency to recur at regular intervals • Things that are periodic:
Mendeleev’s periodic table was so successful because it allowed him to predict the properties of still unknown elements • Eka-Aluminum (Gallium) • Eka-Silicon (Germanium)
Lothar Meyer (1869) • Published almost the same element classification scheme as Mendeleev but did not receive credit because Mendeleev revealed his first and Mendeleev was more successful at demonstrating its usefulness
Henry Moseley • Realized that the periodic table was not in the perfect order • Arranged the modern periodic table. • Listed the elements according to atomic number • Important because: once arranged by atomic number all the elements were in order by their chemical & physical properties • The modern periodic table is listed in order of atomic number
Periodic Law • The physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasing atomic number
Periodic Table • Arrangement of the elements in order of their atomic numbers so that the elements are periodic functions of their atomic numbers.
Element Key: • Includes the element symbol, element name, atomic mass and atomic number • May include other information
Groups (also called Families) • The vertical columns on the periodic table • There are 18 groups, labeled with the numbers 1-18. 1 18 2 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12
Group Names Alkal i Metals Noble Gases Alkal ine Earth Met. Boron Group Carbon Group Nitrogen Group Oxygen Group Halogens Transition Metals Lanthanides Actinides
Periods • Horizontal Rows on the Periodic Table • There are 7 periods labeled with the numbers 1-7. 1 2 3 4 5 6 7
States of Matter (at Room Temp.) Solids: Black lettering on the wall periodic table Liquids: Blue lettering on the wall periodic table (Hg & Br) Gases: Red lettering on the wall periodic table (noble gases, F, Cl, O, N, H)
PROPERTIES OF METALS • Typically solids at room temperature • Good conductors of heat & electricity • High melting points • Luster (shiny) • Malleable (can be hammered into sheets) • Ductile (can be pulled into wires)
Nonmetals • Make up 99% of Earth’s atmosphere (Oxygen & Nitrogen) • Do not conduct electricity and poor conductors of heat • Brittle when solids • Many are gases at room temperature • Lack luster • Low melting points
Nonmetals-Located right of the zig-zag line Exception: hydrogen
Metalloids • Properties of the Metalloids • Have some chemical and physical properties of metals and other properties of nonmetals • Some are semiconductors • Semiconductor: An element that does not conduct electricity as well as a metal but does conduct slightly better than a nonmetal • Computers, Handheld electronic devices, calculators
MetalloidsElements bordered by the zig-zag line (exception: Al is a metal)
Radioactive Elements • Elements with atomic numbers higher than 82 • Radioactivity: Spontaneous emission of radiation • Elements are radioactive because they have too many or too few neutrons • The protons in the nucleus naturally repel each other. The neutrons are the “glue” that hold the nucleus together.
Synthetic Elements • The synthetic elements are the elements with the outlined symbols on the wall periodic table. • Synthetic elements are not found in nature. They are man-made elements.
Periodic Trend • Properties of the elements that change in a predictable way as you move through the periodic table Energy Levels Valence electrons Oxidation Numbers Ionic Size Atomic Size
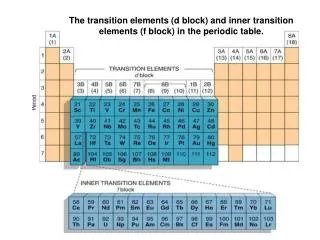
Electron Configurations • Representative Elements: Represent the properties of all of the elements • s-block: Groups 1 & 2 • p-block: Groups 13-18 • Transition Elements: Their valence electrons can transition • d-block: Groups 3-12 • Inner Transition Elements: • f-block: Bottom 2 rows (Lanthanides & Actinides)
Highest Energy Level • The Period # is the same as the atom’s Highest Energy Level • Example: If an element is in Period 6, it’s highest energy level is the 6th energy level 1 Period # is the same as the Highest Energy Level 2 3 4 5 6 7
Valence Electrons • The # of electrons in the highest (outermost) energy level • Transition Metals: The # of valence electrons for a transition metal can vary due to the closeness of their s & d sublevels • Exceptions: • Silver is always 1 valence electron • Zinc is always 2 valence electrons • Inner Transition Metals: Typically have 3 valence electrons
Lewis Dot Diagrams • The element symbol, used to represent the element’s inner level electrons, is surrounded by dots to represent the element’s valence electrons • The # of dots must equal the # of valence electrons, no more than 2 dots per side • Remember: The valence electrons can never be greater than 8, therefore, there can never be more than 8 dots.
Oxidation Number • Equal to the charge assigned to an atom according to electronegativity rules • Ion: Atom that has a charge due to the loss or gain of electrons • Octet Rule: Atoms tend to gain, lose or share electrons so that each atom has a full outermost energy level which is typically 8 valence electrons (octet)
ION • An atom becomes an ion when it gains or loses electrons • The protons in an atom never change—an atom CANNOT gain or lose protons
ION An ion does not have equal numbers of protons and electrons (the positive does not = the negative)…therefore… an ION is a CHARGEDatom ATOM ION
Oxidation Number • If an element loses electrons, its oxidation # is a _______________ number because: there are more positive protons than negative electrons Ca+ion (a positively charged ion) • Elements with 1-3 valence electrons: • Lose electrons to become stable • Form ions with a positive charge
ATOM vs. CATION Positively charged proton Negatively charged electron
Oxidation Number If an element gains electrons, its oxidation # is a _______________ number because: there are more negative electrons than positive protons. A n ion • Elements with 5-7 valence electrons: • Gain electrons to become stable • Form ions with a negative charge • Elements with 4 valence electrons: • Metals will lose electrons, becoming positive ions • Nonmetals will gain electrons, becoming negative ions egative
ATOM vs. ANION Positively charged proton Negatively charged electron
Oxidation Number • Transition Metals • Oxidation #s may vary • Except: Ag+1 & Zn+2 • Inner Transition Metals: • Typically a +3 Oxidation Number
Atomic Radius • Moving across a period, atomic size decreases because: there are more protons attracting electrons in the same energy level • Moving down a group, atomic size increases because: the energy levels increase
Ionic Size The size of an atom when it gains or loses electrons to become stable.
Ionic Size • If an atom loses its valence electrons, its size becomes _____________, because it has lost its highest energy level. • The ion in this case would have a positive charge. • A positive ion would be _________________ than its corresponding atom. • Metals lose electrons easily, therefore, they typically acquire positive charges
Ionic Size • If an atom gains electrons, its size becomes ______________, because there are more electrons than protons and the protons hold the electrons less tightly • The ion in this case would have a negative charge. • A negative ion would be __________________ than its corresponding atom. • ______________ gain electrons easily, therefore, they typically acquire negative charges
IONIZATION ENERGY • Energy needed to remove an electron from an atom
IONIZATION ENERGY • High Ionization Energy: Hard to remove electrons • More energy is needed to remove an electron from an atom that does not want to lose its electrons • Low Ionization Energy: Easy to remove electrons • Less energy is needed to remove an electron from an atom that already wants to lose its electrons
IONIZATION ENERGY As you move across a period, the ionization energy increases because: Moving from metals to nonmetals. Nonmetals do not want to lose electrons. The atom decreases in size moving across the period, therefore, the outer level electrons are closer and more attracted to the nucleus As you move down a group the ionization energy decreases because the outer level electrons are further from the nucleus, and therefore, easier to remove.
IONIZATION ENERGY Trend is opposite of atomic size because the electrons in smaller atoms are held more strongly by the nucleus. Therefore, smaller atoms have higher ionization energies.