Quantum Mechanics
150 likes | 489 Vues
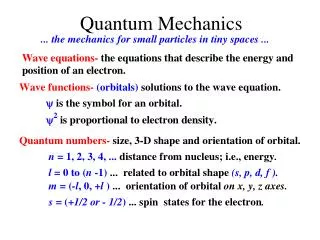
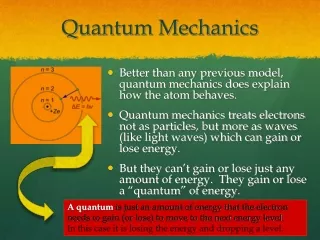
Quantum Mechanics. Quantum Mechanical Model of the Atom. This is the current model of the atom (…ish) Also includes an electron cloud Electrons are moving in waves- not in orderly, straight paths. Electron movement. In orbitals NOT ORBITS Basketball court model

Quantum Mechanics
E N D
Presentation Transcript
Quantum Mechanical Model of the Atom • This is the current model of the atom (…ish) • Also includes an electron cloud • Electrons are moving in waves- not in orderly, straight paths.
Electron movement • In orbitals NOT ORBITS • Basketball court model • In a given region around the nucleus based on energy level
Structure of Energy Levels • Most broad: electrons are in atoms • Within atoms: there are Quantum Levels (n) • Within quantum levels: there are sublevels • S: 2 electrons in 1 orbital • P: 6 electrons in 3 orbitals • D: 10 electrons in 5 orbitals • F: 14 electrons in 7 orbitals • Where are they on the periodic table?
Number of sublevels in each quantum level • Follow the periodic table!! • n = 1 has only s • n = 2 has s and p The quantum levels do not always match up with the rows of the periodic table… • n = 3 has s,p, and d • n = 4 has s,p,d, and f
Notation • Electron configurations! • Break the configurations up by: • n (Quantum Level) • Sublevel (s, p, d, f) • Number of electrons within that sublevel (written as a superscript)
Electron configuration • The distribution of electrons among the sublevels of an atom. • ALWAYS letters and numbers • Fill out total number of electrons for each element shown on your notes page (H through B and Mn)
Orbital Diagrams • Orbital diagrams will always use boxes- these boxes show the orbitals in an atom. • Follow the sublevels, arrows for each electron • Try these: • Oxygen • Chlorine
Rules • Aufbau Principle (snake diagram) • You must fill a sublevel before moving on to the next one. The periodic order must be obeyed! • Pauli Exclusion Principle • A maximum of two electrons can share an orbital. If two electrons share the same orbital, they will have opposite spin. • Hund’s Rule (Roommate rule) • You must put one electron into each orbital before pairing them up.