Predicting Products to Reactions
140 likes | 341 Vues
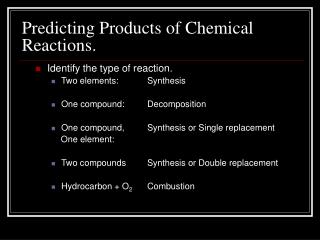
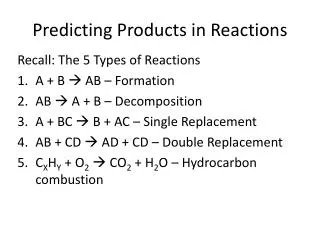
Predicting Products to Reactions. Create this chart. Synthesis Reactions. Example 1: Two elements binary ionic compound 2Na (s) + Cl 2 (g) 2 NaCl (s) Example 2: Water and a small molecular gas acid H 2 O (l) + SO 3 (g) H 2 SO 4 (aq). Decomposition.

Predicting Products to Reactions
E N D
Presentation Transcript
Synthesis Reactions Example 1: Two elements binary ionic compound 2Na (s) + Cl2 (g) 2 NaCl (s) Example 2: Water and a small molecular gas acid H2O (l) + SO3 (g) H2SO4 (aq)
Decomposition Example 1: Binary ionic compound pure elements 2 ZnO (s) 2 Zn (s) + O2 (g) Example 2: Water hydrogen and oxygen gases 2 H2O (l) 2 H2 (g) + O2 (g) Example 3: Hydrogen peroxide water and oxygen gas (needs a kick- light or MnO2) 2 H2O2 (aq) 2 H20 (l) + O2 (g)
Single replacement Pure element and a ionic compound. Check the activity series of metals to see if the reaction will occur or check the order of halogens. I increasing activity F
Examples Al(OH)3 (aq) + 3 Na (s) 3NaOH (aq) +Al (s) Al (OH)3 (aq) + Pb (s) No reaction NaCl (aq) + Br2 (l) No reaction 2 NaCl (aq) + F2 (g) 2NaF (aq) +Cl2 (g)
Double displacement Two aqueous ionic compounds 2 new compounds in which one is a: *precipitate (insoluble compound) or *water or *molecule compound that would be a gas
Examples CaBr2 (aq) + Na2CO3 (aq) 2 NaBr (aq) + CaCO3 (s) NaOH (aq) + HCl (aq) HOH (l) + NaCl (aq) water NaClO3 (aq) + Li NO3 (aq) No reaction because both products are soluble
Combustion Hydrocarbon and oxygen gas water and carbon dioxide gas (complete combustion) 2 C2H6 (g) + 9 O2 (g) 6 H2O (g) + 4 CO2 (g)