Understanding Ionic Compounds: Formation, Properties, and Naming Conventions
430 likes | 604 Vues
Ionic compounds are formed through the transfer of electrons between atoms, leading to the creation of oppositely charged ions that are held together by electrostatic attractions. These compounds are represented by empirical formulas, reflecting their structure as formula units in a crystal lattice. Ionic compounds are electrically neutral, maintaining a balance of positive and negative charges, and possess specific naming conventions based on the oxidation states of their ions. This resource explores the foundational concepts, charge prediction using the periodic table, and naming techniques for both positive and polyatomic ions.

Understanding Ionic Compounds: Formation, Properties, and Naming Conventions
E N D
Presentation Transcript
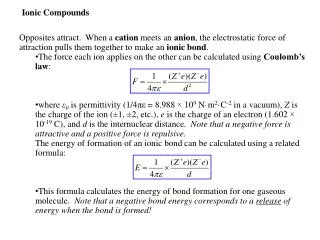
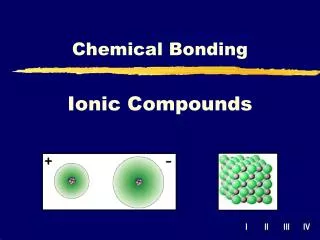
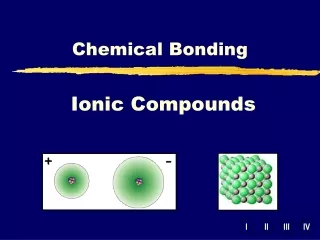
Ionic Compounds When two atoms approach each other the following may happen: 1. A transfer of electron(s) may occur 2. Leads to the formation of oppositely charged ions 3. These oppositely charged ions are held together by electrostatic attraction (Coulombic attraction) This results in an ionic or electrovalent bond between the atoms
Ionic Salts • Since ionic compounds aren't composed of molecules, but instead, are composed of large numbers of formula units found in a lattice (a crystal’s repetitious geometry or array of particles), they are represented by empirical formulas • {What is an empirical formula?} • A formula unit is another means of representing the simplest combining unit of an ionic compound
Charges of Ions • Ions are represented by the chemical symbol of the element with a +/- superscript to indicate the charge on the ion • e.g. F-, Cl-1, Mn+7, K+, Rb1+ • The charge a monatomic ion may exhibit is referred to as the atom's oxidation number (oxidation state) • For some elements this may be established by a set of rules • And, some elements have more than one oxidation number • e.g. transition elements
Combinations of Ions • The valence of an atom is considered its combining ability with other atoms • Ionic compounds have a fixed ratio of cations and anions such that: • The total number of positive charges is equal to the total number of negative charges in a formula unit
Compounds are Neutral • Ionic compounds are electrically neutral - they have a net charge of zero (0) • The formula of ionic compounds is obtained through the manipulation of subscripts to obtain the simplest ratio of equal numbers of positive and negative charges
Predicting the charge of an Ion Utilize the periodic table and knowledge of valence configurations Summary of major states: Group 1 (Family IA) - form (+1) ions Group 2 (Family IIA) - form (+2) ions Group 18 (Family VIIIA) - form 0 ions Group 17 (Family VIIA) - forms (- 1) ions Group 16 (Family VIA) nonmetals - form (- 2) ions Group 15 (Family VA) nonmetals - form (- 3) ions
Writing Ionic Chemical Formulas The positive ion/element is written first • The more positive element is written first in cases of more than one positive element (usually metals) The negative element (usually nonmetal) is written last Subscripts are manipulated to achieve a neutral compound The total positive charges must equal the total negative charges NOTE: subscripts of one (1) are never expressed
Polyatomic Ions • Polyatomic ions are groups of covalently bonded atoms which possess/exhibit an overall electrical charge • These atoms are tightly bonded, and therefore, usually behave and function as a single unit
Polyatomic Ions in Formula Writing • Any time the subscript of a polyatomic ion is greater than one (1) the ion must be placed in parentheses and the subscript placed outside • No parentheses are used when the ion’s subscript is one!! • e.g. magnesium sulfate • Mg(SO4) - incorrect • Mg(SO4)1 - incorrect • Mg1(SO4)1 - incorrect • MgSO4 - correct
Naming Positive Ions The positive component(s) of the compound is/are named first In naming, first determine what is the positive component If it is ammonium, NH4+, write its name If it is a metal that has only one possible oxidation state, write its name **This includes Group 1 (alkali metals), Group 2 (alkaline earth metals), and the elements Al (aluminum), Zn (zinc), Cd (cadmium), and Ag (silver)** There are some others, but this will be the list for now
Positive ions with more than one Oxidation State Traditional naming Traditional naming involves attaching an –ic suffix to the ion of greater charge and an –ous suffix to lesser charge to the root of the element’s name Frequently the Latin roots (roots of origin) are used To memorize: iron – ferrum ferrous ion is Fe+2 and ferric ion is Fe+3 copper – cuprum cuprous ion is Cu+ and cupric ion is Cu+2 tin – stannum stannous ion is Sn+2 and stannic ion is Sn+4 lead – plumbum plumbous ion is Pb+2 and plumbic ion is Pb+4 gold – aurum aurous ion is Au+ and auric ion is Au+3 mercury – hydragyrum mercurous ion is Hg2+2 and mercuric ion is Hg+2 (Note - mercury’s +1 ion is actually found as a diatomic unit!!)
The Stock System Stock naming involves placing a Roman numeral in parentheses after the element’s name to indicate the oxidation state (charge) currently exhibited by that element (ion) e.g. Fe+3 is the iron (III) ion Mn+7 is the manganese (VII) ion
What is the charge on that ion? How to determine the charge on an element from the formula of the compound (or a polyatomic ion) 1. Determine the total negative charge in the compound 2. Change the negative sign to a positive sign – the total positive charge in a compound equals the total negative charge in the compound, as compounds are neutral (no net charge) 3. Divide this positive charge by the number of metal ions in the compound
Example Fe2(SO4)3 3 x SO4-2 = - 6 - 6 => + 6 + 6 / 2Fe = Fe+3 therefore, each ion has a +3 charge iron (III) sulfate or ferric sulfate
Naming Negative Ions Naming the negative component(s); If the negative component is a nonmetal its name must be modified and an – ide suffix attached This is applied to all negatively charged elemental ions e.g. F – fluorine becomes F- - fluoride • P – phosphorus becomes P-3 – phosphide • H – hydrogen becomes H- - hydride
Naming Polyatomic Oxyanions • Looking at the oxyanions – negative polyatomic ions containing oxygen • Note: There are a few exceptions • The most common ion is often from the “– ate” group of ions – memorize these ions
Naming Polyatomic Oxyanions cont’d If the ion’s central atom increases in charge or an oxygen is added, retain the charge and the name of the ion will be “per –” root “– ate” A “per –” prefix is added to the “– ate” form per-__________-ate
Naming Polyatomic Oxyanions cont’d If the ion’s central atom decreases in charge or an oxygen is lost from the “– ate” form of the ion, the name of the ion becomes root “– ite” The “- ate” suffix form becomes an “– ite” suffix form _________ - ite
Naming Polyatomic Oxyanions cont’d If the ion’s central atom decreases in charge or an oxygen is lost from the “– ite” form the ion becomes “hypo –” root “– ite” A “hypo-” prefix is added to the “– ite” form hypo - __________ - ite
Example of Polyatomic Oxyanion • Name Formula Charge on central atom • perchlorate ClO4- Cl +7 • chlorate ClO3 - Cl +5 • chlorite ClO2- Cl +3 • hypochlorite ClO - Cl +1
Molecular Compounds Binary compounds composed of two nonmetals are usually molecular in nature • Rather than observing a transfer of electrons between atoms, these atoms tend to share one or more electron pairs between them • e.g. CH4, NO2, CO2, PCl3 H O = C = O H - C – H H
Nomenclature of Molecular Compounds • Traditional system - involves attaching prefixes to the names of the bonded elements to indicate how many atoms of each element are present in a molecule of the compound Prefixes: mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca If the more positive element’s prefix is mono -, the prefix may be dropped and just the element’s name used The name of the more negative element is still modified and an – ide suffix attached
Special Situation Also, if a two-syllable prefix is to be attached to a name beginning with a vowel, the vowel of the prefix is dropped before attachment Impacts oxygen, iodine, arsenic e.g. pentoxide not pentaoxide
Examples CO2 – carbon dioxide PCl3 – phosphorus trichloride CO - carbon monoxide AsCl5 – arsenic pentachloride
Stock System for Molecular Compounds Stock system – involves the use of Roman numerals in parentheses following the more positive element’s name Recognize, that while no transfer of electrons may occur, an “apparent” positive charge may be assigned to the element of lower electronegativity or lower ionization energy This may be developed due to inequalities in the effective nuclear charges of the bonding atoms
Stock System for Molecular Compounds The apparent positive charge is referred to as its oxidation number and will usually be similar to its ionic character Oxygen is assigned an apparent charge of negative two (- 2) This is then used to assign the oxidation numbers of the other elements
Examples CO2 – carbon (IV) oxide PCl3 – phosphorus (III) chloride CO – carbon (II) oxide AsCl5 – arsenic (V) chloride
Acids The term acid is from acere (Latin) meaning sour Acids have a sour taste, in contrast to bases which have a bitter taste e.g. vinegar or lemon juice Acids will turn litmus red while their counterparts the bases turn litmus blue Litmus is a vegetable material whose color may be altered depending upon the pH of the surrounding environment
More Acid Properties Acids and bases are opposites, they will each destroy the properties of the other referred to as neutralization e.g. KOH(aq) + HCl(aq) ----------> KCl(aq) + H2O(l) Acids and bases will both conduct electrical current Therefore, they are placed along with salts in the group referred to as electrolytes (substances which conduct when placed in an aqueous solution) Acids will react with active metals forming salts and hydrogen gas e.g. Mg(s) + 2 HCl(aq) ---- MgCl2(aq) + H2(g) metal acid “salt” Additonal note - bases also tend to feel slippery (soapy)
Acid Strength Strong acids – these acids completely ionize when placed in solution the [H+] of a strong acid is essentially equal to that acid’s concentration all of the acid molecules dissociates, therefore, no acid molecules actually remain examples of strong acids are hydrochloric, nitric, sulfuric, hydrobromic, hydroiodic, perchloric
Strong Acid’s cont’d these strong acids are considered completely ionized at 1.0 M or less sulfuric is strong only in its first ionization
Weak Acids Weak acids – these acids ionize but to an extent less than 100 % many ionize to less than 10 % of their molecules partial ionization of the acid if an acid isn’t strong, then it will be considered a weak acid
Identifying an Acid Acids represent a group of compounds capable of donating a proton formulas of acids begin with the “active” hydrogen e.g. HCl, HNO3, H3PO4
Naming Binary Acids Binary acids are composed of hydrogen and a nonmetallic element They are named using the form: hydro – nonmetal root – ic e.g. HCl – hydrochloric acid H2S – hydrosulfuric acid HI – hydroiodic acid
Naming Oxyanions Oxyacids contain hydrogen and an oxyanion (polyatomic w/ oxygen) They are named using the form: anion prefix – anion root – ic/-ous (if present) The “–ate” ions become “–ic” acids and the “–ite” ions become “–ous” acids
Examples HNO3 – nitric acid HNO2 – nitrous acid H2SO4 – sulfuric acid H2SO3 – sulfurous acid HClO4 – perchloric acid HClO – hypochlorous acid
Hydrates Many salts interact with water to form compounds having definite numbers of water molecules attached. These compounds are referred to as hydrates or being hydrated. These hydrates often result when a compound is formed through the process of crystallization. The water is referred to as water of crystallization or water of hydration.
Water(s) of Hydration The water of hydration is usually weakly bonded to the salt. As a result, gentle heating is often capable of driving off the water of hydration by vaporization. The substance remaining is the anhydrous salt. • Anhydrous meaning “without water.”
Formulas of Hydrates The formulas for hydrates are expressed as the anhydrous formula followed by a “dot” with a coefficient placed in front of the formula for water to indicate the number of water molecules attached per formula unit of salt. e.g. Na2CO3 • 10 H2O washing soda MgSO4 • 7 H2O epsom salts CuSO4 • 5 H2O blue vitriol of Roman vitriol
Names of Hydrates Naming these hydrates involves writing the name of the anhydrous salt first followed by a prefix attached to the word hydrate. The Greek prefix refers to the coefficient before the formula of water. The word hydrate refers to water.
Examples Referencing the above formulas respectively sodium carbonate decahydrate magnesium sulfate heptahydrate copper (II) sulfate pentahydrate or cupric sulfate pentahydrate