Chemical Reactions Overview: Synthesis, Decomposition, Combustion
140 likes | 232 Vues
Learn about Synthesis, Decomposition, and Combustion reactions in chemistry, with specialized types explained, examples provided, and impact discussed.

Chemical Reactions Overview: Synthesis, Decomposition, Combustion
E N D
Presentation Transcript
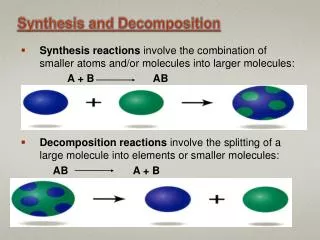
Synthesis • Two species combine to create one • Often, you will see two elements reacting to make a compound Ex: 2Na + Cl2 2 NaCl
Specialized type of Synthesis • Metal Oxides and Water • Produces a metal hydroxide compound (basic) • Ex: Na2O + H2O 2 NaOH • Non-metal Oxides and Water • Produces a solution that is acidic • Acid Precipitation • Ex: SO2 + H2O H2SO3
Decomposition • One species breaks down into at least two different species • Often, this will be a compound breaking down into elements Ex: 2 H2O 2H2 + O2
Specialized Types of Decomposition • Bicarbonates (hydrogen carbonates) • Carbon dioxide gas is formed • Other products: carbonate and water vapour • Ex: 2 KHCO3 K2CO3 + H2O + CO2 • Carbonates • Under extreme heating conditions • Produces an oxide and carbon dioxide gas • Ex: K2CO3 K2O + CO2
Nitrates • When a metal nitrate decomposes, it forms a metal nitrite and oxygen gas • Ex: 2 KNO3 2 KNO2 + O2 5. Hydroxides • When a metal hydoxide decomposes, it forms a metal oxide and water • Ex: Mg(OH)2 MgO + H2O
Combustion • An element or compound is reacting with oxygen, accompanied by a release of energy as heat or light • Products will always include an oxide • Hydrocarbons: made of carbon and hydrogen • Ex: propane (C3H8), gasoline, methane (CH4)…
Complete Combustion: efficient combustion with ample amounts of oxygen • Flames are blue, indicating a higher temperature • Products: Carbon dioxide and water • Ex: CH4 + 2 O2 CO2 + 2H2O
Incomplete combustion: combustion when there is not enough oxygen • Flames are orange, indicating a “cooler” temperature • Products are varied: can include carbon (soot) and carbon monoxide (which are both pollutants), plus carbon dioxide and water • Ex: 2 C3H8 + 7O2 2C + 2CO + 2CO2 + 8 H2O
Combustion of other chemicals • When other chemicals undergo combustion, other oxides are created • Ex: NO2 and SO3 (non-metal oxides react with water - dangerous for the environment) • Combustion of Hydrogen gas -http://www.youtube.com/watch?v=nLuOM9aOWvk
Homework • P. 121 # 4, 5, 7 • Worksheet