Calculating Average Atomic Mass
70 likes | 247 Vues
Calculating Average Atomic Mass. Unit 9 worksheet 2. Average atomic mass. The decimal number on the periodic table The weighted average of all the isotopes of an element Depends on the percent (relative) abundance and the mass of each isotope Measured in “atomic mass units” (amu). Problem 1.

Calculating Average Atomic Mass
E N D
Presentation Transcript
Calculating Average Atomic Mass Unit 9 worksheet 2
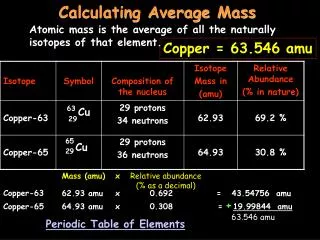
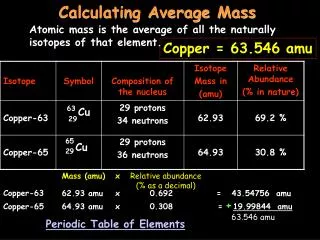
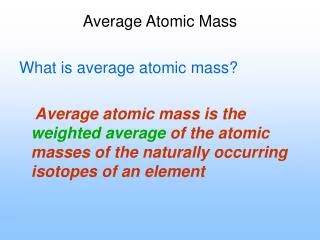
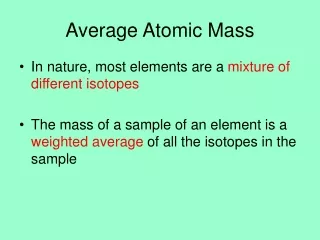
Average atomic mass • The decimal number on the periodic table • The weighted average of all the isotopes of an element • Depends on the percent (relative) abundance and the mass of each isotope • Measured in “atomic mass units” (amu)
Problem 1 • Given: • Element X has 2 isotopes • Mass = 6 amu and percent abundance = 7.5% (0.075) • Mass = 7 amu and percent abundance = 92.5% (0.925)
To solve Average atomic mass = [(mass) x (abundance)] + [(mass) x (abundance)] [(6) X (0.075)] + [(7) x (0.925)] 0.45 + 6.475 6.925 amu On the periodic table, this is closest to Li, atomic number 3.