Solution Stoichiometry
250 likes | 852 Vues
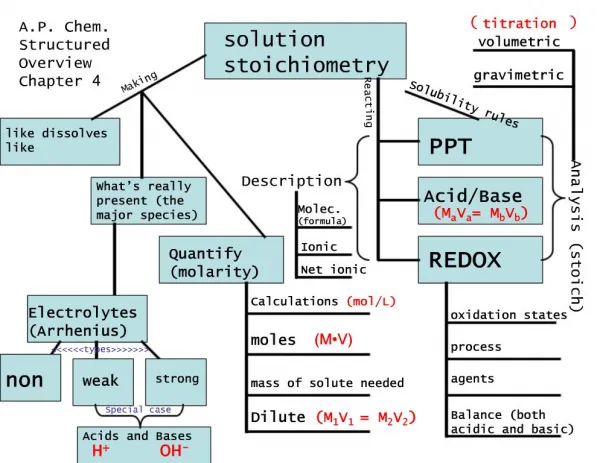
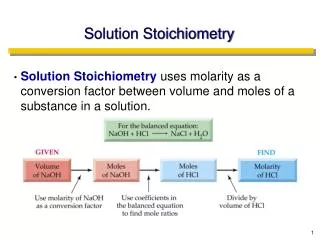
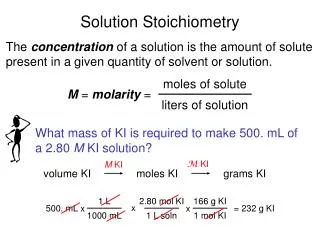
Solution Stoichiometry. Solution. mixture homogeneous 2 or more components (solvent and solute) composition varies within limits Concentration amount of solute dissolved in a given amount of solution independent of quantity. Molarity (molar concentration). PLAN:.

Solution Stoichiometry
E N D
Presentation Transcript
Solution • mixture • homogeneous • 2 or more components (solvent and solute) • composition varies within limits • Concentration • amount of solute dissolved in a given amount of solution • independent of quantity
PLAN: Molarity is the number of moles of solute per liter of solution. 1.80mol HBr 1000mL 455 mL soln 1 L PROBLEM: Hydrobromic acid(HBr) is a solution of hydrogen bromide gas in water. Calculate the molarity of hydrobromic acid solution if 455mL contains 1.80mol of hydrogen bromide. SOLUTION: = 3.96M
How many moles of KI are in 84 mL of 0.50 M KI solution? • 0.42 mol
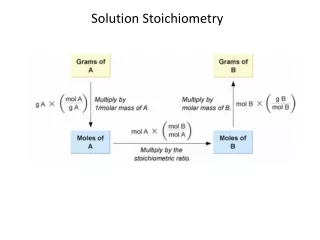
MASS (g) of compound in solution AMOUNT (mol) of compound in solution MOLECULES (or formula units) of compound in solution VOLUME (L) of solution Figure 3.12 Summary of mass-mole-number-volume relationships in solution. M (g/mol) Avogadro’s number (molecules/mol) M (g/mol)
0.460moles 1 L 141.96g Na2HPO4 mol Na2HPO4 PROBLEM: How many grams of solute are in 1.75L of 0.460M sodium monohydrogen phosphate? SOLUTION: 1.75L = 0.805mol Na2HPO4 0.805mol Na2HPO4 = 114g Na2HPO4
In biochemistry laboratories, solutions of sucrose (table sugar, C12H22O11) are used in high-speed centrifuges to separate the parts of a biological cell. How many liters of 3.30 M sucrose solution contain 135 g of solute? • M of sucrose = 342 • moles = 0.395 • V = 0.120 L
A • Weigh the solid needed. • Transfer the solid to a volumetric flask that contains about half the final volume of solvent. CAdd solvent until the solution reaches its final volume. BDissolve the solid thoroughly by swirling. Laboratory preparation of molar solutions. Figure 3.12
How many grams of Na2SO4 are required to make 0.350 L of a 0.500 M solution? • moles req = 0.175 • M = 142 • mass = 24.9 g
Figure 3.13 Converting a concentrated solution to a dilute solution.
Dilution • from a more concentrated stock solution MdilxVdil = #mol solute = MconcxVconc
0.15 mol NaCl L soln L solnconc 6 mol Sample Problem 3.14 Preparing a Dilute Solution from a Concentrated Solution PROBLEM: “Isotonic saline” is a 0.15 M aqueous solution of NaCl that simulates the total concentration of ions found in many cellular fluids. Its uses range from a cleaning rinse for contact lenses to a washing medium for red blood cells. How would you prepare 0.80 L of isotonic saline from a 6.0 M stock solution? PLAN: It is important to realize the number of moles of solute does not change during the dilution but the volume does. The new volume will be the sum of the two volumes, that is, the total final volume. MdilxVdil = #mol solute = MconcxVconc volume of dilute soln SOLUTION: multiply by M of dilute solution moles of NaCl in dilute soln = mol NaCl in concentrated soln 0.80 L soln = 0.12 mol NaCl divide by M of concentrated soln 0.12 mol NaCl = 0.020 L soln L of concentrated soln
If 25.0 mL of 7.50 M sulfuric acid are diluted to exactly 500. mL, what is the mass of sulfuric acid per mL in the new solution? • new conc = 0.375 M • 0.375 mol per L; 36.75 g/L = 0.03675 g/mL
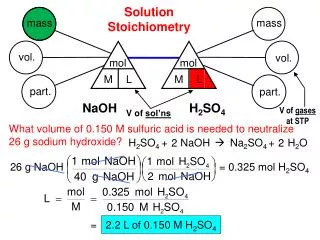
mass Mg(OH)2 L HCl divide by M divide by M mol Mg(OH)2 mol HCl mol ratio PROBLEM: Specialized cells in the stomach release HCl to aid digestion. If they release too much, the excess can be neutralized with antacids. A common antacid contains magnesium hydroxide, which reacts with the acid to form water and magnesium chloride solution. As a government chemist testing commercial antacids, you use 0.10M HCl to simulate the acid concentration in the stomach. How many liters of “stomach acid” react with a tablet containing 0.10g of magnesium hydroxide? PLAN: Write a balanced equation for the reaction; find the grams of Mg(OH)2; determine the mol ratio of reactants and products; use mols to convert to molarity.
Mg(OH)2(s) + 2HCl(aq) MgCl2(aq) + 2H2O(l) mol Mg(OH)2 58.33g Mg(OH)2 2 mol HCl 1 mol Mg(OH)2 1L 0.10mol HCl SOLUTION: 0.10g Mg(OH)2 = 1.7x10-3 mol Mg(OH)2 1.7x10-3 mol Mg(OH)2 = 3.4x10-3 mol HCl 3.4x10-3 mol HCl = 3.4x10-2 L HCl
How many grams of Ca(OH)2 are required to react completely with (neutralize) 25.0 mL of 0.100 M HNO3?Ca(OH)2 + 2HNO3→ Ca(NO3)2 + 2H2O • moles HNO3 = 2.50 x 10-3 • M of Ca(NO3)2 = 74.1 • mass = 0.0926 g
Sample Problem 3.16 Solving Limiting-Reactant Problems for Reactions in Solution PROBLEM: Mercury and its compounds have many uses, from fillings for teeth (as an alloy with silver, copper, and tin) to the industrial production of chlorine. Because of their toxicity, however, soluble mercury compounds, such mercury(II) nitrate, must be removed from industrial wastewater. One removal method reacts the wastewater with sodium sulfide solution to produce solid mercury(II) sulfide and sodium nitrate solution. In a laboratory simulation, 0.050L of 0.010M mercury(II) nitrate reacts with 0.020L of 0.10M sodium sulfide. How many grams of mercury(II) sulfide form? PLAN: As usual, write a balanced chemical reaction. Since this is a problem concerning a limiting reactant, we proceed as we would for a limiting reactant problem. Find the amount of product which would be made from each reactant. Then choose the reactant that gives the lesser amount of product.
Hg(NO3)2(aq) + Na2S(aq) HgS(s) + 2NaNO3(aq) L of Hg(NO3)2 L of Na2S multiply by M multiply byM mol Hg(NO3)2 x 1mol HgS mol Na2S 1mol Na2S mol ratio x 1mol HgS mol ratio 1mol Hg(NO3)2 mol HgS mol HgS 232.7g HgS 1 mol HgS Sample Problem 3.16 Solving Limiting-Reactant Problems for Reactions in Solution continued SOLUTION: 0.050L Hg(NO3)2 0.020L Hg(NO3)2 x 0.010 mol/L x 0. 10 mol/L = 5.0x10-4 mol HgS = 2.0x10-3 mol HgS Hg(NO3)2 is the limiting reagent. 5.0x10-4 mol HgS = 0.12g HgS
Figure 3.15 An overview of the key mass-mole-number stoichiometry relationships.
PROBLEM: Mercury and its compounds have many uses, from filling teeth (as an alloy with silver, copper, and tin) to the industrial production of chlorine. Because of their toxicity, however, soluble mercury compounds, such mercury(II) nitrate, must be removed from industrial wastewater. One removal method reacts the wastewater with sodium sulfide solution to produce solid mercury(II) sulfide and sodium nitrate solution. In a laboratory simulation, 0.050L of 0.010M mercury(II) nitrate reacts with 0.020L of 0.10M sodium sulfide. How many grams of mercury(II) sulfide form?
Hg(NO3)2(aq) + Na2S(aq) HgS(s) + 2NaNO3(aq) x 1mol HgS 1mol Na2S x 1mol HgS 1mol Hg(NO3)2 232.7g HgS 232.7mol HgS SOLUTION: 0.050L Hg(NO3)2 0.020L Hg(NO3)2 x 0.010 mol/L x 0. 10 mol/L = 5.0x10-4 mol HgS = 2.0x10-3 mol HgS Hg(NO3)2 is the limiting reagent. 5.0x10-4 mol HgS = 0.12g HgS