Programmed Cell Death
20 likes | 328 Vues
Programmed Cell Death. SIGMA-ALDRICH. Programmed Cell Death

Programmed Cell Death
E N D
Presentation Transcript
Programmed Cell Death SIGMA-ALDRICH
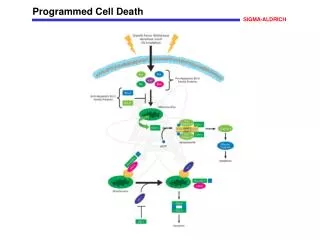
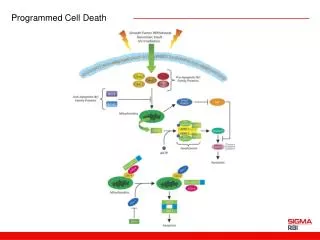
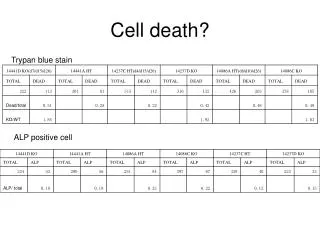
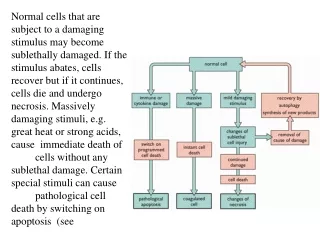
Programmed Cell Death Programmed cell death (PCD), or apoptosis, can be triggered by a wide range of stimuli, including cell surface receptors like Fas and FasL. It constitutes a system for the removal of unnecessary, aged, or damaged cells that is regulated by the interplay of proapoptotic and antiapoptotic proteins of the Bcl-2 family. The proapoptotic proteins Bax, Bad, Bid, Bik, and Bim contain an -helical BH3 death domain that fits the hydrophobic BH3 binding pocket on the antiapoptotic proteins Bcl-2 and Bcl-XL, forming heterodimers that block the survival-promoting activity of Bcl-2 and Bcl-XL. Thus, the relative abundance of proapoptotic and antiapoptotic proteins determines the susceptibility of the cell to programmed death. The proapoptotic proteins act at the surface of the mitochondrial membrane to decrease the mitochondrial trans-membrane potential and promote leakage of cytochrome c. In the presence of dATP cytochrome c complexes with and activates Apaf-1. Activated Apaf-1 binds to downstream caspases, such as procaspase-9, and processes them into proteolytically active forms. This begins a caspase cascade resulting in apoptosis. References Kelekar, A., and Thompson, C.B., Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell. Biol., 8, 324-330 (1998). Priault, M., et al., Investigation of bax-induced release of cytochrome c from yeast mitochondria: permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur. J. Biochem., 260, 684-691 (1999). McDonnell, J.M., et al., Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell, 96, 625-634 (1999).