anion gap metabolic acidosis
250 likes | 1.02k Vues
Metabolic acidosis. Overproduction or ingestion of fixed acid or loss of base which produces an increase in arterial pH (an acidemia)HCO3 is used to buffer the extra fixed acid.As a result, the arterial HCO3 decreases. Acidemia causes hyperventilation (Kussmaul breathing), which is the respiratory compensation for metabolic acidosis..

anion gap metabolic acidosis
E N D
Presentation Transcript
1. Anion gap metabolic acidosis More then just a mud pile
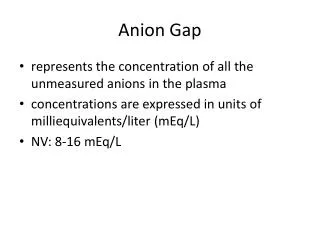
3. The anion gap An estimate of the unmeasured anions.
Used to determine if a metabolic acidosis is due to an accumulation of non-volatile acids (e.g. lactic acid) OR a net loss of bicarbonate (e.g. diarrhea)
Anion gap = Na � (Cl + HCO3)
4. The influence of albumin Albumin is a major source of unmeasured anions!
If a patient�s serum albumin is low, then the patient has more unmeasured anions then the anion gap predicts.
Corrected AG = Observed AG + 2.5 x (4.5 � measured albumin)
5. More then one problem? The �gap-gap� or �delta-delta�
In the presence of a high AG metabolic acidosis, it is possible to detect another metabolic acid base disorder by comparing the AG excess to the HCO3 deficit
Delta-Delta = (Measured AG � 12)/(24-measured HCO3)
6. Mixed Disorders When a fixed acid accumulates in extracelluar fluid, the decrease in serum HCO3 is equivalent to the increase in AG and the gap-gap ratio = 1
When a hypercholemic acidosis appears, the decrease in HCO3 is greater then the increase in AG, and the gap-gap <1 (i.e. coexistent metabolic acidosis)
When alkali is added in presence of high AG acidosis, the decrease in bicarbonate is less then increase in AG and the gap-gap > 1 (i.e. coexistent metabolic alkalosis)
7. Differential for AG Metabolic Acidosis Ketoacidosis
Lactic acid acidosis
Toxin-induced metabolic acidosis
Renal failure acidosis
8. Ketosis Occurs in conditions of reduced nutritient intake, adipose tissues release free fatty acids, which are taken up in the liver and metabolized to form the ketones, acetoacetate and B-hydroxybutyrate.
The ACETEST a nitroprusside reaction detects acetoacetate NOT hydroxybutyrate.
9. Ketosis Diabetic ketoacidosis
Alcoholic ketoacidosis
Starvation ketosis
10. Alcoholic Ketoacidosis Some chronic alcoholics, esp binge drinkers, who discontinue solid food intake while continuing EtOH consumption develop this form of ketoacidosis when EtOH ingestion is curtailed abruptly.
Metabolic acidosis may be severe but is accompanied by only a modest derangement in glucose levels (usually low but may be slightly elevated).
11. Alcoholic Ketoacidosis Presentation may be complex because a mixed disorder is often present
Metabolic alkalosis from emesis
Respiratory alkalosis from EtOH liver disease
Lactic acid acidosis from hypoperfusion
Therapy includes IV glucose and saline
Check electrolytes frequently
High potential for refeeding syndrome
12. Lactic Acid Acidosis Lactic acid can exist in two forms: L-lactate and D-Lactate. In mammals, only the levorotary form is a product of metabolism.
D-Lactate can accumulate in humans as a byproduct of metabolism by bacteria, which accumulate and overgrow in the GI tract with jejunal bypass or short bowel syndrome.
The lab measures only L-lactate!
13. L-Lactic Acidosis Tissue underperfusion (Type A)
Shock, shock, shock
Hypoxia
Asthma
CO poisoning
Severe anemia
14. L-Lactic Acidosis Medical conditions (w/o tissue hypoxia)
Hepatic failure
Thiamine deficiency (co-factor for pyruvate dehyrogenase)
Malignancy
Bowel ischemia
Seizures
Heat stroke
Tumor lysis
Drugs/Toxins
Metformin (particulary associated with hypovolemia and dye)
NRTI (especially stavudine and zidovudine)
Propofol
Nitroprusside
15. L-Lactic Acidosis Propylene Glycol toxicity
An alcohol used to enhance water solubility of many hydrophobic IV medications (lorazepam, diazepam, esmolol, nitroglycerin)
Propylene glycocol toxicity from solvent accumulation has been reported in 19% to 66% of ICU patients receiving high dose lorazepam or diazepam for more then 2 days.
Signs of toxicity�agitation, coma, seizures, tachycardia, hypotension
16. Toxic-Induced Metabolic Acidosis Salicylates
More common in children then in adults
May result in high AG metabolic acidosis
Most commonly associated with respiratory alkalosis due to direct stimulation of the respiratory center
17. Osmolar Gap Under most physiologic conditions, Na, urea and glucose generate the osmotic pressure of blood .
Serum OSM = 2 (Na) + BUN/2.8 + glc/18
Calculated and determined OSM should agree within 10 to 15 mOsm/kg.
If not, then serum Na may be spuriously low OR osmolytes other then Na, glc or urea have accumulated.
The osmolar gap is a reliable and helpful tool when screening for toxin-associated high AG acidosis.
18. Toxic-Induced Metabolic Acidosis Ethanol
In general does not cause high AG metabolic acidosis
Oxidized to acetaldehyde, acetyl CoA and CO2
Acetaldehyde levels increase significantly if acetaldehyde dehydrogenase inhibited by disulfiram, insecticides or a sulfonurea.
Paraldehyde
Very rare
19. Toxic-Induced Metabolic Acidosis Methanol
Causes metabolic acidosis in addition to severe optic nerve and CNS manifestations
High osmolar gap
Ethylene Glycol
Leads to high AG metabolic acidosis in addition to severe CNS, cardiopulmonary and renal damage.
Recognizing oxalate crystals in urine facilitates diagnosis.
High osmolar gap
20. Uremia At a GFR < 20 mL/min, the inability to excrete H+ with retention of acid anions such as phosphate and sulfate results in an increased anion gap acidosis, which RARELY is severe.
The unmeasured anions �replace� bicarbonate (which is consumed as a buffer).
Hyperchloremic normal anion gap acidosis develops in milder cases of renal insufficiency.
21. References Marino, P. 2007. The ICU Book. 3rd Edition. Philadelphia. Lippincott.
Brenner and Rector. 2007. The Kidney. 8th Edition. New York. Saunders.
McPhee S andPapadakis M. 2007. Current Medial Diagnosis and Treatment. New York. McGraw-Hill.
Up to Date 2008.