Gas Stoichiometry Problems Solved: Moles to Liters Conversion
110 likes | 230 Vues
Solve gas stoichiometry problems converting moles to liters of a gas at Standard Temperature and Pressure (STP) or non-STP using the ideal gas law. Examples and step-by-step calculations provided. Courtesy Christy Johannesson.

Gas Stoichiometry Problems Solved: Moles to Liters Conversion
E N D
Presentation Transcript
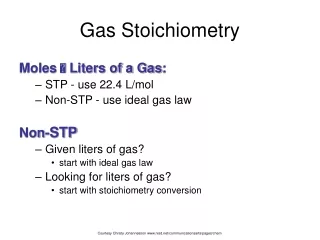
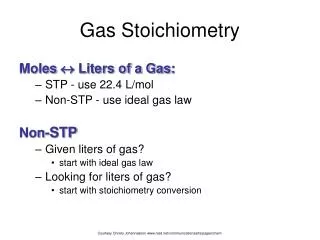
Gas Stoichiometry Moles Liters of a Gas: • STP - use 22.4 L/mol • Non-STP - use ideal gas law Non-STP • Given liters of gas? • start with ideal gas law • Looking for liters of gas? • start with stoichiometry conversion Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
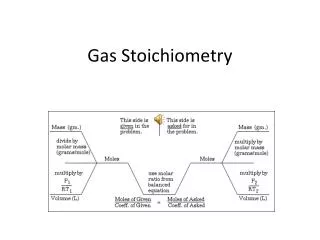
Gas Stoichiometry Problem What volume of CO2 forms from 5.25 g of CaCO3 at 103 kPa & 25ºC? CaCO3 CaO + CO2 5.25 g ? Lnon-STP Looking for liters: Start with stoich and calculate moles of CO2. 5.25 g CaCO3 1 mol CaCO3 100.09g CaCO3 1 mol CO2 1 mol CaCO3 = 1.26 mol CO2 Plug this into the Ideal Gas Law to find liters. Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Gas Stoichiometry Problem • What volume of CO2 forms from 5.25 g of CaCO3 at 103 kPa & 25ºC? GIVEN: P = 103 kPa V = ? n = 1.26 mol T = 25°C = 298 K R = 8.315dm3kPa/molK WORK: PV = nRT (103 kPa)V=(1mol)(8.315dm3kPa/molK)(298K) V = 1.26 dm3 CO2 Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Gas Stoichiometry Problem How many grams of Al2O3 are formed from 15.0 L of O2 at 97.3 kPa & 21°C? 4 Al + 3 O2 2 Al2O3 15.0 L non-STP ? g GIVEN: P = 97.3 kPa V = 15.0 L n = ? T = 21°C = 294 K R = 8.315dm3kPa/molK WORK: PV = nRT (97.3 kPa) (15.0 L)= n (8.315dm3kPa/molK) (294K) n = 0.597 mol O2 Given liters: Start with Ideal Gas Law and calculate moles of O2. NEXT Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Gas Stoichiometry Problem How many grams of Al2O3 are formed from 15.0 L of O2 at 97.3 kPa & 21°C? 4 Al + 3 O2 2 Al2O3 15.0L non-STP ? g Use stoich to convert moles of O2 to grams Al2O3. 0.597 mol O2 2 mol Al2O3 3 mol O2 101.96 g Al2O3 1 mol Al2O3 = 40.6 g Al2O3 Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
n R T V = P Gas Stoichiometry Find vol. hydrogen gas made when 38.2 g zinc react w/excess hydrochloric acid. Pres. = 107.3 kPa; temp.= 88oC. Zn (s) + 2 HCl (aq) ZnCl2(aq) + H2(g) 38.2 g excess X L P = 107.3 kPa (13.1 L) T = 88oC 1 mol H2 22.4 L O2 1 mol Zn x L H2 = 38.2 g Zn = 13.1 L H2 1 mol Zn 1 mol H2 65.4 g Zn Zn H2 Combined Gas Law At STP, we’d use 22.4 L per 1 mol, but we aren’t at STP. 1 mol H2 1 mol Zn x mol H2 = 38.2 g Zn = 0.584 mol H2 1 mol Zn 65.4 g Zn 88oC + 273 = 361 K 0.584 mol (8.314 L.kPa/mol.K)(361 K) P V = n R T 16.3 L = = 107.3 kPa
Gas Stoichiometry Find vol. hydrogen gas made when 38.2 g zinc react w/excess hydrochloric acid. Pres. = 107.3 kPa; temp.= 88oC. Zn (s) + 2 HCl (aq) ZnCl2(aq) + H2(g) 38.2 g excess X L P = 107.3 kPa (13.1 L) T = 88oC 1 mol H2 22.4 L O2 1 mol Zn x L H2 = 38.2 g Zn = 13.1 L H2 1 mol Zn 1 mol H2 65.4 g Zn Zn H2 Combined Gas Law At STP, we’d use 22.4 L per 1 mol, but we aren’t at STP. P1 = T1 = V1 = P2 = T2 = V2 = 101.3 kPa (101.3 kPa) x (13.1 L) = (107.3 kPa) x (V2) P1 x V1 T1 P2 x V2 T2 = 273 K 273 K 361 K 13.1 L 107.3 kPa 16.3 L V2 = 88 oC + 273 = 361 K X L
P V n = R T What mass solid magnesium is required to react w/250 mL carbon dioxide at 1.5 atm and 77oC to produce solid magnesium oxide and solid carbon? 2 + CO2 (g) 2 MgO (s) + C (s) Mg (s) 250 mL 0.25 L X g Mg 0.25 L V = 250 mL oC + 273 = K T = 77oC 350 K P = 1.5 atm 151.95 kPa (0.250 L) 151.95 kPa 1.5 atm = 0.013 mol CO2 n = P V = n R T (350 K) 0.0821 L.atm / mol.K 8.314 L.kPa / mol.K 24.3 g Mg 2 mol Mg x g Mg = 0.013 mol CO2 = 0.63 g Mg 1 mol Mg 1 mol CO2 CO2 Mg
Gas Stoichiometry How many liters of chlorine gas are needed to react with excess sodium metal to yield 5.0 g of sodium chloride when T = 25oC and P = 0.95 atm? 2 2 Na + Cl2 NaCl excess X L 5 g 1 mol NaCl 1 mol Cl2 22.4 L Cl2 x g Cl2 = 5 g NaCl = 0.957 L Cl2 58.5 g NaCl 2 mol NaCl 1 mol Cl2 P1 x V1 T1 P2 x V2 T2 Ideal Gas Method = P1 = 1 atm T1 = 273 K V1 = 0.957 L P2 = 0.95 atm T2 = 25 oC + 273 = 298 K V2 = X L (1 atm) x (0.957 L) (0.95 atm) x (V2) = 273 K 298 K V2 = 1.04 L
n R T V = P Gas Stoichiometry How many liters of chlorine gas are needed to react with excess sodium metal to yield 5.0 g of sodium chloride when T = 25oC and P = 0.95 atm? 2 2 Na + Cl2 NaCl excess X L 5 g 1 mol NaCl 1 mol Cl2 x g Cl2 = 5 g NaCl = 0.0427 mol Cl2 58.5 g NaCl 2 mol NaCl Ideal Gas Method P V = n R T P = 0.95 atm T = 25 oC + 273 = 298 K V = X L R = 0.0821 L.atm / mol.K n = 0.0427 mol 0.0427 mol (0.0821 L.atm / mol.K) (298 K) X L = 0.95 atm V = 1.04 L