Antibody Structure and Function
750 likes | 2.49k Vues
Antibody Structure and Function. W. Robert Fleischmann, Ph.D. Department of Urologic Surgery University of Minnesota Medical School rfleisch@umn.edu (612) 626-5034. Objectives. Understand the basic structure and function of antibodies Understand how antigens and antibodies interact

Antibody Structure and Function
E N D
Presentation Transcript
Antibody Structure and Function W. Robert Fleischmann, Ph.D. Department of Urologic Surgery University of Minnesota Medical School rfleisch@umn.edu (612) 626-5034
Objectives • Understand the basic structure and function of antibodies • Understand how antigens and antibodies interact • Understand the role of antibodies in protection from toxins, bacteria, viruses and parasites • Understand the mechanisms by which antibodies provide this protection
The Purpose of Adaptive Immunity • Innate immunity deals with infectious agents when we first come into contact with them. • Adaptive immunity deals with infectious agents, toxins, and foreign cells • that persist in our body for an extended period of time or • that enter our body a second time.
Components of Adaptive Immunity • Adaptive immunity has two components. • Humoral immunity is mediated by B cells and plasma cells. • Cell-mediated immunity is mediated by CD8+ cytotoxic T cells. • Adaptive immunity must be carefully regulated. • Under-stimulation of adaptive immunity can fail to generate sufficient memory to last a lifetime. • Over-stimulation of adaptive immunity can lead to the development of autoimmunity or blood cell cancers.
Development of Humoral Immunity • Monocytes, dendritic cells, and other professional antigen-presenting cells present a specific antigen to a subset of CD4+ helper T cells (Th2 cells) that carry a T cell receptor that recognizes (binds to) that specific antigen. • Antigen-presenting cells provide additional signals to activate Th2 cells. • Antigen-presenting cells and activated Th2 cells interact with B cells that recognize the specific antigen. • Antigen-presenting cells present specific antigen to B cells that carry an antibody on their surface that recognizes that specific antigen. • Th2 cells secrete cytokines that activate B cells. • B cells divide and differentiate to become antibody-producing plasma cells.
IL-2 IL-3 IFN- CD4 Th1 CD8 T cell IL-1 IL-12 M Cytolysis: FAS/FAS ligand TNF Granzyme B Perforin Antibody CD4 Th2 IL-1 Summary of Acquired Immunity IL-4 IL-5 IL-6 IL-9 IL10 IL-13 B cell/Plasma cell
Antigens: Definition • Antigens are infectious agents, toxins, and foreign cells that contain unique molecular structures that can be recognized by humoral immunity. • Antigens are recognized by T cell receptors and by antibodies. • Antigens may be proteins, carbohydrates or lipids, though the best antigens are proteins.
Antigens: Characteristics • Antigens have one or more immune recognition sites called epitopes. • The more complex the antigen, the more epitopes it has.
Epitopes: Characteristics • Epitopes are generally hydrophilic regions of an antigen. • Epitopes recognized by B cells are linear or conformational regions located on the surface of the antigen that are available for recognition and binding by antibody. • Epitopes recognized by T cells are linear regions of an antigen that have been cut from the antigen and presented to the T cell receptor in association with an MHC molecule. • Linear epitopes are 5-7 aa in size. • Confirmational epitopes may include discontinuous aa groups spread over a 15-22 aa sequence. • Epitopes vary in their ability to stimulate an immune response. • Immunodominant epitopes induce a greater immune response than other epitopes on an antigen.
Visualization of Epitopes • Epitopes are regions on an antigen that can be recognized by the paratope (antigen-binding site) of an antibody or by a T cell receptor. • Epitopes are also called antigenic determinants. • In the picture, epitopes on a whole poliovirus virion and on the isolated poliovirus VP1 protein are shown in white. • As can be seen for the individual VP1 protein in the bottom picture, a given protein can have multiple epitopes.
Biological Molecules Have Differing Immunogenicity • Thymus-Dependent Antigens • Proteins are the most immunogenic. • Thymus-Independent Antigens • Polysaccharides are potentially immunogenic. • ABO blood groups. • Nucleic Acids are poorly antigenic unless bound to protein carriers. • Anti-DNA antibodies are seen in Lupus and other auto-immune diseases. • Lipids are not usually antigenic unless bound to proteins. • Thymus-independent antigens involve recognition of conformational epitopes.
Haptens: a Special Class of Small Antigens • Some molecules are too small to stimulate an immune response but can still bind to antibody. • Thus, these small molecules are antigens but not immunogens. • Such molecules are called haptens. • Haptens can stimulate immunity when combined (covalently or noncovalently) with a suitable carrier molecule. • Example: penicillin allergy, where penicillin is the hapten
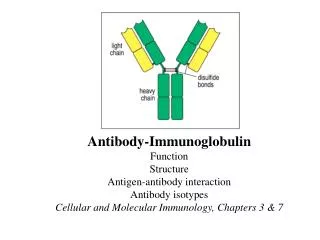
Antibodies Are Members of the Immunoglobulin Supergene Family • Other members of the immunoglobulin supergene family (IgSF) include a variety of cell adhesion proteins • ICAM, VCAM, T cell receptor, MHC molecules, CD4, CD8, and CD28 (binds to B7), IL-1 receptor • Members of the IgSF have one or more immunoglobulin domains • Two or more -pleated sheets arranged in opposite directions that are stabilized by one or more disulfide bonds (aka -barrel)
Albumin Globulins Absorbance Migration distance Antibodies: Definition • Antibodies are members of a family of proteins that are collectively known as immunoglobulins (Igs). • Antibodies are present in the third peak of electrophoretically separated serum globulin proteins (that are heavier than albumin), hence they are also called “gamma” globulins. • All antibodies have similar structural features.
Albumin Globulins Absorbance Migration distance Multiple Myeloma • Antibodies are produced by B cells and plasma cells. • When a single plasma cell becomes transformed into a cancerous cell, it causes myeloma. • Myeloma patients over-produce a homogeneous Ig produced by a single plasma cell. This can be observed as a heightened peak of Ig by electrophoresis of blood proteins. • Myeloma patients also have some immunoglobulin proteins that spill over into their urine. These Bence-Jones proteins are dimers of kappa or lambda light chains. IgX from Myeloma patients
Antibodies: General Features • Antibodies recognize unique regions on antigens called epitopes. • Each antibody recognizes a single epitope by its unique paratope or antigen-binding site. • The sum of all of the epitopes recognized by all of the different antibodies that a person can produce is called the person’s repertoire. • Every individual has their own repertoire. • Some individuals respond better to certain epitopes and therefore to certain antigens than to others.
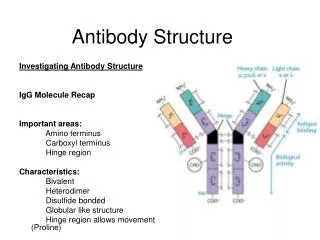
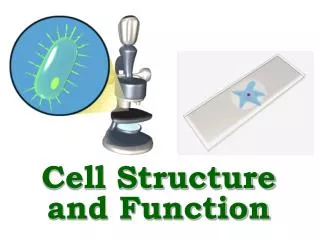
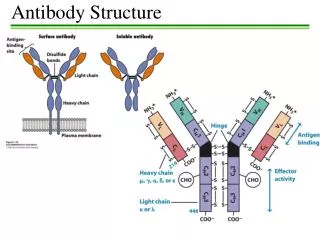
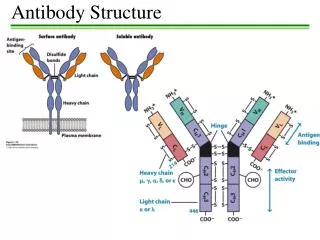
Antibody Structure • Antibodies are hetero-tetramers of four peptide chains. • Antibodies contain two identical light chains and two identical heavy chains. • Each light chain has a molecular weight of about 22,000 daltons. • Each heavy chain has a molecular weight of about 55,000 daltons. • The total molecular weight of an antibody is about 150-160 daltons.
Light chain (kappa or lambda) Heavy chain (isotypic region: , , , or) General Structure of an Antibody Light chains: Kappa chains are found twice as frequently in Abs as are lambda chains. Heavy chains: These undergo class switching as the B cell undergoes differentiation.
Light Chains • MWs of light chains are about 22,000 daltons (about 200 amino acids). • From study of Bence-Jones proteins of different multiple myeloma patients: • The amino terminal half of the proteins varied from patient to patient; e.g. variable region. • The carboxy terminal half of the proteins varied among two groups; constant region. • 60% are chains, with no subtypes • 40% are chains, with 1, 2, 3, and 4 subtypes differing in only a few amino acids • Antibody contains two copies of the same light chain
Heavy Chains • MWs of heavy chains are about 55,000 daltons (about 500 amino acids). • From heavy chains of antibodies of different myeloma patients: • The amino terminal 20% of the proteins varied greatly from patient to patient; e.g. variable region. • The carboxy terminal part of the proteins varied primarily among two groups: and chains. • We now know that there are five types of heavy chain • IgD, containing the heavy chain • IgM, containing the heavy chain • IgG, containing the heavy chain, with 1, 2, 3, 4 subtypes • IgA, containing the heavy chain, with 1 and 2 subtypes • IgE, containing the heavy chain • Antibody contains two copies of the same heavy chain which may pair with either type of light chain
Molecular Model of IgG AntigenBinding LightChain Heavy Chain Carbohydrate
Fab and Fc Regions of an Antibody Fab = variable region aka VDJ region aka idiotypic region aka paratope region Light chain (kappa or lambda) Heavy chain (isotypic region: , , , or) Fc = constant region • Papain cleaves just above the double disulfide bond, giving 2 Fab fragments and 1 Fc fragment. • Pepsin cleaves just below the double disulfide bond and within the constant region, giving 1 Fab2 fragment and degraded Fc.
Fc Versus Fab and Fab2 • Fab and Fab2 fragments get their name because they contain one or two antigen binding sites of the antibody. • The Fc fragment gets its name because it was noted to crystallize from solution when chilled.
Fab Versus Fab2 • Fab cannot precipitate antigen, because it has only one antigen combining site and can combine with only one antigen epitope. • Fab2 can precipitate antigen, because it has two antigen combining sites and can combine with two antigen epitopes (cross-linking antigens).
Functions of Antibody Defined by Studies of Fab and Fc Regions • Fab regions: Antigen binding to antigenic epitopes occurs at the variable regions of the Fab, located at the amino terminal regions of the heavy and light chains. • Fc region: Various effector activities occur at the constant regions, located at the carboxy terminal regions of the heavy chains. • Binding to Fc receptors on macrophages • Binding to and activation of complement factor C1 (C1qr2s2) (requires conformational change that occurs with binding of Ag to Fab region of Ab)
The Immunoglobulin Domain • Immunoglobulins possess several homologous Ig domains of about 110 aa, stabilized by a disulfide bond. • Light chain has 1 variable domain and 1 constant domain. • Heavy chain has 1 variable domain and 3 or 4 constant domains. • Either 3 constant domains and a hinge structure as is seen for , , and heavy chains. (Hinge structure gives more flexibility in the two arms.) • Or 4 constant domains without a hinge structure for and heavy chains.
Stylized Domain Structure • The Ig Domain has an intra-domain loop of about 60 aa held together by a disulfide bond. Kuby Immunology, 6th Edition
Structure of an Ig Domain • The Ig domains are folded into a characteristic structure called the immunoglobulin fold. • The immunoglobulin fold is a “sandwich” composed of two -pleated sheets containing alternating antiparallel strands connected by loops of varying length. This gives a relatively rigid structure to the immunoglobulin folds. • Note that the blue loops of the variable region form three complementarity-determining regions(CDRs) that together form the antibody’s paratope or antigen-binding site (epitope). Constant domain Variable domain CDR CDR Kuby Immunology
Complementarity Determining Regions (CDRs) • There are 3 CDRs on the light chain and 3 CDRs on the heavy chain. • The CDRs are also called hypervariable regions because they show the greatest amount of variability from antibody to antibody. • The fact that they are on the loops of the Ig domain indicate that, while they may have slight flexibility, they are held in place by a relatively rigid structure.
Antigen Binding to Antibody Kuby Immunology
Characteristics of Ag-Ab Binding • The binding of antigen to antibody is a non-covalent interaction that involves • Electrostatic forces (salt bridges; ionic bonds) • Hydrogen bonds • Van der Waals forces • Hydrophobic forces • Although each individual interaction is weak, the binding strength of an antigen to its antibody is due to the sum of a large number of low energy interactions. • Thus, the binding between the paratope of the antibody and its antigen epitope can be very strong (dissociation constant 10-7-10-10). Kuby, Immunology
Antibody Avidity • This is the sum of all of the affinity interactions between a given antigen and its antibody. • Depends upon the binding affinity of each paratope for its cognate epitope. • Depends upon the number of identical combining sites (epitopes) on an antigen. Some antigens may have a single epitope for a given paratope. • Depends upon the number of combining sites (paratopes) on an antibody. For example, IgG has two paratopes while IgM has ten. Thus, even if an individual IgM paratope had five-fold less affinity than an IgG paratope, they could have equal avidity (if there were 10 identical epitopes on the antigen).
Antigen Binding Induces a Conformational Change in the Antibody • Binding of the antigen epitope to the antibody’s paratope causes changes the conformation of the antibody. • Antibody with no bound antigen does not bind complement, while antibody with bound antigen does bind complement. • Much like the change in enzyme conformation by the binding of its substrate.
Advantages of the Hinge Region • In the , , and heavy chains of IgG, IgD, and IgA, there is a hinge region between the first and second constant domains. • This allows wide or narrow separation of the two arms of the antibodies. • Can form interactions between paratopes on different arms of an antibody and epitopes on different antigens. • If the antigen has multiple epitopes, the antigen:antibody complexes can form large cross-linked structures (latice-like structures). • The large cross-linked structure can then precipitate because they become too large to stay in solution. Note: IgM antibody can precipitate antigens because it has a multimeric structure. Soluble IgE does not precipitate antigens, as it is bound to mast cells and basophils. Also, cross-linking of IgE antibodies on mast cells and basphils is key to degranulation.
Cross-Reactivity of Antigens • Some antigens have within them the same sequence of amino acids and thus share a common epitope. • Cross-reactivity can also be seen as a slight mismatch of epitope and paratope, that differ by a similar amino acid (for example, ala vs. leu). • Medical application: Antibodies to Group A streptococci (Streptococcus pyogenes) cross-react with certain proteins in the heart values and can cause post-streptococcal rheumatic fever.
Site of Antibody Expression • Most types of antibodies are expressed as soluble antibodies that have been secreted into blood, lymph, or bodily secretions such as saliva, tears, milk, intestinal secretions, and urogenital secretions. • Some types of antibodies are expressed on the surface of immune cells. • IgD is bound to B cells • Some forms of IgM are bound to B cells; others are soluble • IgE is bound to mast cells and basophils
Antibody Functions • Mediation of uptake and processing of antigens • Neutralization of antigens • Opsonization of antigens • Agglutination of antigens • Precipitation of antigens • Activation of complement • Evocation of antibody-dependent cell-mediated cytotoxicity • Establishment of passive immunity via transfer in placenta and colostrum
Uptake And Processing of Ag • B cells expressing IgD or IgM bind antigen. • The Ag:Ab complex is internalized. • The antibody is degraded or recycled and the antigen is digested (processed) to peptides in phagolysosomes. • Specific peptide sequences (epitopes) of the antigen are bound to MHC class II and presented to helper T cells to trigger either more antibody synthesis or cytotoxic T cell activation.
Neutralization of Antigens • Certain epitopes on antigens are involved in antigen binding to target cells or molecules. • If the antigen is a toxin, the antibody is called an antitoxin. • Serum containing antitoxins is called antiserum. • Antibodies that recognize and bind to these epitopes block the ability of the antigen to bind to the target cell or molecule. • This can keep bacteria from being able to adhere to tissue cells. • The antigen is said to be neutralized.
Opsonization of Antigens • Binding of antibodies to antigens can facilitate the uptake of the antigens by phagocytic cells (opsonization). • The antibodies bind to antigens by the Fab portion of the antibody, leaving the Fc portion of the antibody free to bind to Fc receptors on the surface of phagocytic cells. • The antibodies bind via Fc: Fc receptor binding and the antigen is phagocytosed.
Agglutination of Antigens • Individual antigens can be bound by multiple antibodies, either recognizing the repeated or different epitopes. • If multiple antibodies bind to a given antigen and if an antibody binds to two or more antigens, the antigens can be clumped together or agglutinated. • Agglutinated antigens can be more readily phagocytosed than individual antigens.
Precipitation of Antigens • When the concentration of soluble antigens and antibodies are about equal, large Ag:Ab complexes can form (agglutination). • If the Ag:Ab complex is sufficiently large, it can precipitate from solution. • Precipitated Ag:Ab complexes can be more readily removed by phagocytes. • However, if precipitated Ag:Ab complexes are not phagocytosized quickly, complement can be activated and damage can occur to the site of the precipitated Ag:Ab complexes (cell surface or basement membrane). • Example: post-streptococcal glomerulonephritis
Activation of Complement • If cross-linking of Ag occurs with Ab binding, complement can be activated. • If complement is activated by antibody binding to a bacterium, lysis of the bacterium will occur due to the deposition of the complement C9 attack complex. • If complement is activated by Ag:Ab that has precipitated on a host cell or basement membrane, deposition of the complement attack complex can damage the cell or basement membrane. • A number of host factors block deposition or activation of complement on normal tissues (eg, protectin [CD59] and homlogous restriction factor [HRF]). • These host factors can be overwhelmed by high levels of activated complement (post-streptococcal glomerulonephritis).
Ab:Ag Activation of Complement:The Classical Pathway of Complement Activation Kuby et al
Three Mechanisms of Complement Activation Mannose-binding lectin Kuby Immunology
Antibody-Dependent Cell-Mediated Cytotoxicity • When Ag:Ab bind and when the Fc portion of the antibody binds to Fc receptors on Natural Killer cells, eosinophils, neutrophils, or macrophages, ADCC can be activated. • ADCC results in the death of bacteria, viruses, parasites, and tumor cells. • The specificity for target cell killing in ADCC is provided by the antibody, not by the effector cell. • ADCC differs from opsonization as the antigen is killed without first being phagocytosed.