The Structure of an Atom
190 likes | 366 Vues
This chapter delves into the fundamental subatomic particles: protons, neutrons, and electrons. Protons are positively charged particles located in the nucleus, while neutrons are neutral counterparts found alongside them. Electrons orbit the nucleus, carrying a negative charge. The chapter compares these particles based on their mass and charge, illustrating how they contribute to the atomic makeup. Additionally, it explores concepts like atomic number, mass number, and isotopes, highlighting their significance in defining elements and understanding atomic behavior.

The Structure of an Atom
E N D
Presentation Transcript
The Structure of an Atom Chapter 4.1
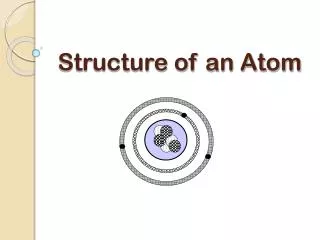
Subatomic Particles 1. Subatomic particles include protons, electrons, and neutrons. 2. Protons A. A proton is a positively charged subatomic particle that is found in the nucleus of an atom. B. Each nucleus must contain at least one proton. C. Each proton is assigned a charge of 1+. D. Some nuclei contain more than 100 protons.
Subatomic Particles 3. Electrons A. An electron is a negatively charged subatomic particle that is found in the space outside the nucleus. B. Each electron has a charge of 1-. 4. Neutrons A. A neutron is a neutral subatomic particle that is found in the nucleus of an atom. B. It has a mass almost exactly equal to that of a proton.
Comparing Subatomic Particles 5. Comparing Subatomic Particles A. Protons, electrons, and neutrons can be distinguished by mass, charge and location in an atom. B. Protons and neutrons have almost the same mass. C. According to the relative mass of electrons in the above table; how many electrons would it take to equal the mass of a proton or neutron? (1/1836)x=1; About 2,000 electrons
Comparing Subatomic Particles D. Electrons have a charge that is equal in size but opposite the charge of a proton. E. Neutrons have no charge. F. Where are protons and neutrons found? • In the nucleus G. Where are electrons found? • The space outside the nucleus.
Comparing Subatomic Particles H. Everything scientists know about the nucleus and subatomic particles is based on how the particles behave. We are still unable to see the inside of an atom. However, we do have microscope that can show how atoms are arranged on the surface of a material.
Atomic/Mass Number 6. Atomic Number and Mass Number A. The atomic number of an element equals the number of protons in an atom of that element. B. The atoms of any given element always have the same number of protons. C. There is one proton in the nucleus of every hydrogen atom. What is the atomic number for hydrogen? • 1
Atomic/Mass Number D. Atoms of different elements have different numbers of protons. E. What other element have an atomic number of 1? • None, only hydrogen has an atomic number of 1. F. Atomic numbers can be used to refer to elements since they are unique to that element.
Atomic/Mass Number G. Each positive charge in an atom is balanced by a negative charge because atoms are neutral. H. The atomic number of an element also equals the number of electrons in an atom.
Mass Number 7. Mass Number A. The mass number of an atom is the sum of the protons and neutrons in the nucleus of that atom. B. You can find the number of neutrons by subtracting the atomic number from the mass number. C. number of neutrons = mass number - atomic number
Isotopes 8. Isotopes A. Isotopes are atoms of the same element that have different numbers of neutrons and different mass numbers. B. Isotopes of an element have the same atomic number but different mass numbers because they have different numbers of neutrons.
Isotopes Ex. 1) Oxygen has 8 protons and 8 neutrons with a mass number of 16. An isotope of oxygen may have 8 protons and 9 neutrons with a mass number of 17.
Isotopes C. These isotopes are referred to by their mass number. Ex. 2) Oxygen-16, Oxygen-17 D. Since hydrogen-1 has no neutrons and one proton adding a neutron doubles its mass. E. Water that contains hydrogen-2 atoms in place of hydrogen-1 atoms is called heavy water