Alpha and Beta Interactions
241 likes | 581 Vues
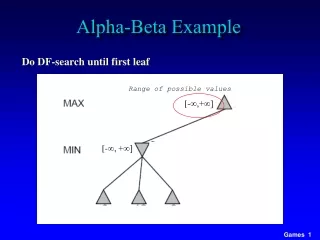
Alpha and Beta Interactions. Rad Pro III NUCP 2331. Interactions. How radiation interacts with matter depends on Energy Mass Charge Charged particles interact differently than uncharged. Attenuation.

Alpha and Beta Interactions
E N D
Presentation Transcript
Alpha and Beta Interactions Rad Pro III NUCP 2331
Interactions • How radiation interacts with matter depends on • Energy • Mass • Charge • Charged particles interact differently than uncharged
Attenuation • Attenuation- process by which the intensity of a radiation beam is reduced as it passes through matter • Can be either • Particulate • Electromagnetic • Direct • indirect
Alpha Particles • Charged particle emitted from the nucleus as a component of decay • 2 protons and 2 neutrons • Emitted with a discrete amount of energy • Emitted from large nucleii • Daughter product has Z-2 and A-4 of parent • Can predict what your daughter will be if one knows the decay method
Alpha characteristics • +2 charge • Atomic mass of 4 • Travel in straight lines • Highly ionizing • Deposits a lot of energy short distance • Easily shielded • Internal hazard • Can interact directly or indirectly • Does it interact more or less as energy increases?
Alpha interactions • Direct collisions with other particles • Electric fields of charged particles will interact as well • If particle has high velocity the time it will interact with other particle is very small • As it slows down the time it interacts with other particle increases , increasing the probability of ionization
Alpha interactions • The, now free, electrons that were ionized by the alpha particle can now go and ionize other atoms • These ionized particle that are generated by these interactions are called delta rays • Delta rays can cause secondary ionization in matter
SPECIFIC IONIZATION Specific ionization for a 4.8 MeV alpha particle in air is 40,000 ion pairs /cm
SHIELDING • Alpha Radiation • Alpha radiation will be stopped by very thin absorbing materials, dead layer of skin, paper • Range in air in cm: (about .7 cm/MeV) R = 0.318 (E3/2) • The ratio of the density of air to the density of any material times the range of the alpha particle in air will give you the range of the alpha particle in that material
Alpha range • Compare formula with chart for • 2 MeV • 5MeV • 7 MeV
CHANGE IN LET WITH INCREASING PATH LENGTH For Heavy Charged Particles Bragg Peak Relative LET Distance of Penetration
Beta Particle • Charged particle emitted from the nucleus that has mass of an electron and can be either – or + 1 • Beta – is emitted from nucleus that has too many neutrons • Neutrons decays into a proton and beta - • Beta+ is emitted form nucleus that has too many protons • Proton sucks up a close electron and turns into a neutron
Beta Characteristics • -1 charge (or +1) • 1/1860 mass of proton • Travel in very curvy path • Atomic number after Beta - decay increases by 1 • Atomic number after Beta + decay decreases by 1 • Less ionizing than alpha more than gamma • Still easily shielded (not as easily as alpha)
Beta • Range = 0.526E - 0.094 E> 0.8 MeV • g/cm2 • Divided by density of material will give range • About 3 m in air/Mev • Shielded by tin, Al, plastic • Internal hazard, • semi-infinite cloud • Compare to chart
SPECIFIC IONIZATION Specific ionization for a 4.8 MeV beta particle in air is 37 ion pairs/cm
SHIELDING • Beta Radiation • The beta particle (electron) produces much less ionization in a given path than the alpha • The beta range curve will work for all materials if one corrects for density • The thickness and choice of shield material depends upon energy and Bremsstrahlung
SHIELDING • Bremsstrahlung • Secondary photon radiation produced by the acceleration of charged particles passing through matter • Bremsstrahlung losses (radiative losses) increases with increasing atomic number (Z) and beta energy
Electromagnetic wave Beta Particle Bremsstrahlung
BremsstrahlungCalculation • Fraction of Beta energy that is converted to gamma ray energy • F= K Z Emax • K= 3.5 E-4 (low Z shields) 5 E-4 (high Z shields) • Z= Atomic number of shield material • Emaz= Max energy of the beta particle
BremsstrahlungCalculation • Have P-32 • 1.7 MeV beta • Compare the amount of energy released as Bremsstrahlung from shielding materials of • Al • Pb