Metals and Non-Metals
730 likes | 3.25k Vues
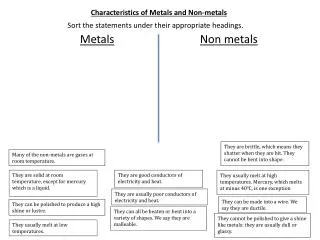
Metals and Non-Metals. Properties of Metals. Almost all metals on the periodic table that are lustrous, malleable, ductile and are good conductors of heat and electricity. Examples:. Properties of Metals cont’d…. Appear on the left side of the periodic table.

Metals and Non-Metals
E N D
Presentation Transcript
Properties of Metals • Almost all metals on the periodic table that are lustrous, malleable, ductile and are goodconductors of heat and electricity. • Examples:
Properties of Metals cont’d… • Appear on the left side of the periodic table. • Metals generally have a high density and high melting point. • Most metals are solid at room temperature except??
Uses of Metals • Because they are good conductors of heat and electricity, metals are very useful. • Examples: • Copper wire used in electrical circuits. • Iron used in pots and pans. • Aluminum used as a heat sink in computers. • Tungsten used in light bulbs.
Properties of Non-Metals • Non-metals tend to have low lustre (dull), be brittle and are not ductile or malleable. • Most are poor conductors of heat and electricity. • Examples:
Properties Cont’d… • Non-metals can be either solids or gases at room temperature, but one is a liquid, which one??
Uses of Non-Metals • Because they are poor conductors of heat and electricity, non-metals can also be very useful. • Example: • Argon gas used as an insulator between panes of glass in windows. • Neon and phosphorus can be used as light sources.
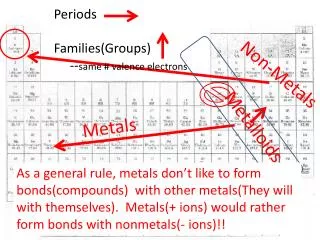
Metals and Non-Metals • Looking at the periodic table in the back of the text book: • Metals are coloured BLUE • Non-Metals are coloured YELLOW • What about the GREEN ones?
Metalloids • Some elements found on the periodic table have properties of both metals and non-metals • These elements are called metalloids. • Examples:
Where are the Metalloids? • Represented by a downward staircase on the periodic table, starting at boron and ending at polonium.
Investigation 5-B: Physical Properties of Metals and Non-Metals • Purpose– what is being investigated? (copy from text) • Hypothesis – answer the question in the text
Analysis – answer question #1 Conclusion – answer questions #2, #3 Type your report and follow the guidelines on the LAB REPORT FORMAT handout. Due Date: Monday, March 3