Intro to Bonding: Ionic Compounds (Type 1 and 2 Binary Compounds)
100 likes | 303 Vues
Intro to Bonding: Ionic Compounds (Type 1 and 2 Binary Compounds). What is a bond?. A bond is a force that holds groups of two or more atoms together. For Example: Water (H 2 O) A water molecule is held together by two bonds: a bond between both H-O. Types of bonds.

Intro to Bonding: Ionic Compounds (Type 1 and 2 Binary Compounds)
E N D
Presentation Transcript
Intro to Bonding:Ionic Compounds(Type 1 and 2 Binary Compounds)
What is a bond? • A bond is a force that holds groups of two or more atoms together. • For Example: • Water (H2O) • A water molecule is held together by two bonds: a bond between both H-O
Types of bonds • There are 3 types of bonds that we are going to focus on: • Ionic Bonds • Covalent Bonds • Polar • Nonpolar • Metallic Bonds
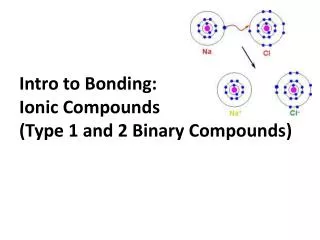
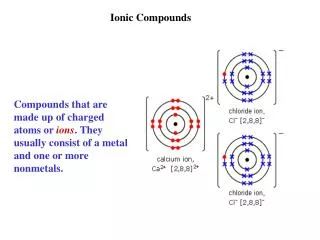
Ionic Bonds • A strong bonding force resulting from the attraction of oppositely charged ions. • Ionic compounds result when a metal reacts with a nonmetal. • An ionic compound contains a cation (metal) and an anion (nonmetal). • Example: • Sodium chloride (NaCl) • Sodium (Na+) is the cation (metal) • Chloride (Cl-) is the anion (nonmetal)
Ionic Bonds • The metal loses an electron to form a cation. • The nonmetal gains that electron from that metal to form an anion. • The result is an ionic bond and the formation of an ionic compound. • The ionic bond is an attraction between ions.
Ionic Bonds - Cations • If we look at the periodic table, the metals are colored in green. The metals lose electrons to form positively charged cations.
Ionic Bonds - Cations • Cation charge can be determined by looking at how the periodic table is organized. • The alkali metals have a +1 charged cation because they donate 1 electron. • The alkaline earth metals have a +2 charged cationbecause they donate 2 electrons. • Metals in column 13 have a +3 charged cation because they donate 3 electrons.
Ionic Bonds - Transition Metal Cations • Transition Metals have multiple charges • For example: • Lead can be Pb+2 or Pb+4 • Copper can be Cu+1 or Cu+2 • Gold can be Au+1 or Au+3 • Iron can be Fe+2 or Fe+3 • and the list goes on…
Ionic Bonds – Anions • If we look at the periodic table, the nonmetals are colored in purple. The nonmetals gain electrons to form negatively charged anions.
Ionic Bonds - Anions • Anion charge can be determined by looking at how the periodic table is organized. • The halogens have a -1 charged anion because they are able to accept 1 electron. • Nonmetals in column 16 have a -2 charged anion because they are able to accept 2 electrons. • Nonmetals in column 15 have a -3 charged anion because they are able to accept 3 electrons.