Three-Dimensional Symmetry
270 likes | 689 Vues
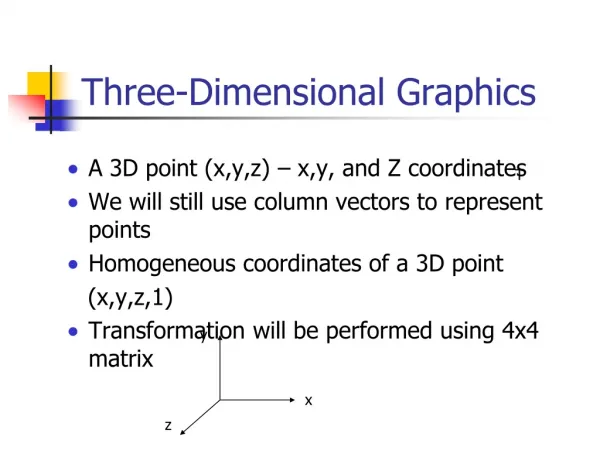
Three-Dimensional Symmetry. How can we put dots on a sphere?. The Seven Strip Space Groups. Simplest Pattern: motifs around a symmetry axis (5) Equivalent to wrapping a strip around a cylinder. Symmetry axis plus parallel mirror planes (5m). Symmetry axis plus perpendicularmirror plane (5/m).

Three-Dimensional Symmetry
E N D
Presentation Transcript
Three-Dimensional Symmetry How can we put dots on a sphere?
Simplest Pattern: motifs around a symmetry axis(5)Equivalent to wrapping a strip around a cylinder
Symmetry axis plus mirror planes and perpendicular 2-fold axes (5m2)
Axial Symmetry • (1,2,3,4,6 – fold symmetry) x 7 types = 35 • Only rotation and inversion possible for 1-fold symmetry (35 - 5 = 30) • 3 other possibilities are duplicates • 27 remaining types
Isometric Symmetry • Cubic unit cells • Unifying feature is surprising: four diagonal 3-fold symmetry axes • 5 isometric types + 27 axial symmetries = 32 crystallographic point groups • Two of the five are very common, one is less common, two others very rare
Non-Crystallographic Symmetries • There are an infinite number of axial point groups: 5-fold, 7-fold, 8-fold, etc, with mirror planes, 2-fold axes, inversion, etc. • In addition, there are two very special 5-fold isometric symmetries with and without mirror planes. • Clusters of atoms, molecules, viruses, and biological structures contain these symmetries • Some crystals approximate these forms but do not have true 5-fold symmetry, of course.
Why Are Crystals Symmetrical? • Electrostatic attraction and repulsion are symmetrical • Ionic bonding attracts ions equally in all directions • Covalent bonding involves orbitals that are symmetrically oriented because of electrostatic repulsion
Why Might Crystals Not Be Symmetrical? • Chemical gradient • Temperature gradient • Competition for ions by other minerals • Stress • Anisotropic surroundings
Regardless of Crystal Shape, Face Orientations and Interfacial Angles are Always the Same
Projections in Three Dimensions are Vital for Revealing and Illustrating Crystal Symmetry