Li
30 likes | 325 Vues
Covalent molecular gases. These elements occur as diatomic (two atom) molecules with strong covalent bonds between the atoms (intramolecular bonds) and weak van der Waal’s forces between the molecules (intermolecular bonds). The weak van der Waals forces mean low melting and boiling points.

Li
E N D
Presentation Transcript
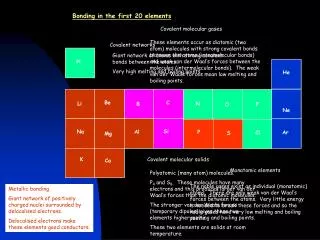
Covalent molecular gases These elements occur as diatomic (two atom) molecules with strong covalent bonds between the atoms (intramolecular bonds) and weak van der Waal’s forces between the molecules (intermolecular bonds). The weak van der Waals forces mean low melting and boiling points. Covalent networks Giant network of atoms with strong covalent bonds between the atoms. Very high melting and boiling points. H He N F O Be C Li Ne B Cl Si P Na Al Ar S Mg Covalent molecular solids K Ca Monatomic elements Polyatomic (many atom) molecules. P4 and S8. These molecules have many electrons and this produces larger van der Waal’s forces than the diatomic molecules. The stronger van der Waals forces (temporary dipoles) gives these two elements higher melting and boiling points. These two elements are solids at room temperature. The noble gases exist as individual (monatomic) atoms. There are only weak van der Waal’s forces between the atoms. Very little energy is needed to break these forces and so the noble gases have very low melting and boiling points. Metallic bonding. Giant network of positively charged nuclei surrounded by delocalised electrons. Delocalised electrons make these elements good conductors. Bonding in the first 20 elements H He Be C N Li F Ne B O Si P Na Al Cl Ar S Mg K Ca
Diagram shows part of the covalent network of carbon atoms in diamond. Each carbon atom is covalently bonded to 4 other carbon atoms. Uneven distribution of the electrons in the electron cloud create temporary dipoles (d+ and d-) which result in a weak attraction between atoms which come close to each other. These weak attractions are called van der Waals’ forces. d+ d- d+ d- H He N F O Be Ne C Li B Cl Ar Si P Na Al S Mg Diagram shows S8 molecules in sulphur with the van der Waals forces shown by the dotted lines. These large molecules have stronger van der Waals forces than the diatomic molecules. Strong covalent bonds between the atoms inside the diatomic molecules. K Ca - - - - - - - + + + + + + + + + + + + + + + + + + + + + Metallic bonding with a network of positively charged nuclei surrounded by a ‘sea’ of delocalised electrons. Weak van der Waal’s forces between molecules which come close to each other. - - - - - - - - - - H He Be Ne C N Li F B O Ar Si P Na Al Cl S S Mg K Ca