Intro to Modern Physics
950 likes | 1.44k Vues
Intro to Modern Physics. Light - Wave or Particle????. Is light only a wave?. http://video.google.com/videoplay?docid=-4237751840526284618#. Review - 19 th Century. Experiments that prove light’s a wave. Interference - "Double Slit Experiment " - constructive/ destructive .

Intro to Modern Physics
E N D
Presentation Transcript
Intro to Modern Physics Light - Wave or Particle????
Is light only a wave? • http://video.google.com/videoplay?docid=-4237751840526284618#
Review - 19th Century Experiments that prove light’s a wave
Interference - "Double SlitExperiment" - constructive/ destructive • This was first shown in 1801 by Thomas Young, who sent sunlight through two narrow slits and showed that an interference pattern could be seen on a screen placed behind the two slits.
2)Polarization - "Filtering of Light - • Un-polarized light is composed of light oscillating in all directions. • Polarizers convert un-polarized light into polarized light that is parallel to the axis of the polarizer.
(20th century) Experiments supporting particle theory of light
Photoelectric Experiment • Experiment in the late 1800's baffles scientists– light was not acting like a wave! • - When light is exposed to the surface of certain metals, the light energy is absorbed andelectrons are ejected • http://www.stmary.ws/highschool/physics/home/animations3/modernPhysics/photoelectricEffect.swf
Photoelectric effect Emitted electrons are called PHOTOELECTRONS Light (or other electromagnetic radiation) Photosensitive metal The light’s energy causes the emission of electrons
Unexpected Results As long as you are above a certain frequency (threshold frequency, f0)... • When you brighten the light source - Get more electron emission Rate of Photoelectronemission Intensity of light (Brightness) …
… BUT…not faster (more energetic) electrons!!!! K.E. ofPhotoelectrons Intensity of light (Brightness) Kinetic Energy of Photoelectrons remains constant Results contradict Wave Theory!
According to Wave Theory • Increasing brightness, increases the energy of the wave • Therefore - Should Increase Kinetic Energy of Electrons Emitted • It Didn't!!
Black -Body Radiation and Planck’s Hypothesis • When a solid is heated it emits electromagnetic radiation • As the temp is increased, the radiation shifts toward shorter wavelengths • i.e. Heated solids glow red, then orange, and finally white • Ideal black body or cavity radiator is a hollow solid with a small opening drilled in one of the walls – used to study black-body radiation
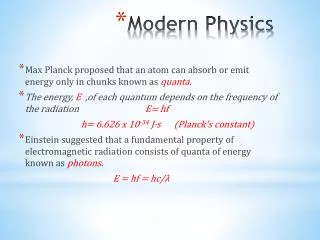
What did they find? • When solid was heated, the radiation emitted through the opening only depended on temperature not material • Why? • Heating caused the atoms to oscillate and then the energy was released as EM radiation • In 1900, Max Planck found that the atomic ‘oscillators’ can only have certain quantized energies
Photons and quantized energy • In 1905, Albert Einstein published a theory explaining the photoelectric effect using quantum theory developed by Max Planck. • Einstein said that light and other electromagnetic radiation consist of discrete, quantized bundles of energy – PHOTONS • The energy of a photon depends on its frequency.
Energy of a Photon • The energy of a quantum is given by the equation: E = h f = h c/λ • E is in Joules • f is in Hz • Planck’s constant, h , is a universal constant h = 6.63 x 10-34 Js • Small energy values of quanta are often expressed in eV 1eV = 1.60 x 10-19 J
homework • In TEXT book • READ pgs 722-734 • Do Practice problems 1-9 all
Einstein’s Photoelectric Equation KEmax = hf – W • When a photon with energy hf strikes a surface, a part of its energy (the work function, W) frees the electron from its bonds. • Remainder energy gives electron its KE
Example • A photon with frequency = 8.0 x 1014hz strikes a photoemissive surface with work function = 1.7 x 10-19 J. Calculate: (a) the maximum KE of ejected electrons (b) the threshold frequency of the surface
Solution (a) KEmax = hf – W = (6.63 x 10-34 Js)(8.0 x 1014hz) – 1.7 x 10 -19 J = 3.6 x 10 -19 J (b) The threshold frequency (f0) is the frequency at which KE of photoelectrons is zero. KEmax = hf – W = 0 So W = h f0 1.7 x 10 -19 J = (6.63 x 10 -34 Js) f0 f0 = 2.6 x 10 14 hz
2nd proof of photon theory The compton Effect
Compton effect • Arthur Compton, a US physicist, bombarded a block of graphite with x-rays of known frequency • He discovered that both electrons and x-rays emerged from the block as shown and found • Scattered x-ray freq, f’ < f , incident x-ray freq and both energy and momentum were conserved Graphite block Scattered x-ray (frequency = f’) θ1 Incident X-ray (frequency = f) θ2 Scattered electron (velocity = v)
Magnitude of momentum of a photon • Photon‘s energy & momentum decrease equalsElectron's energy & momentum increase. **Mass, energy, momentum is conserved. ** p = E/c = hf/c = h/λ Note: High frequency light (uv, x-rays, gamma radiation) behaves more like particles and less like waves; Low frequency light (radio waves, microwaves, infrared radiation) behaves more like waves and less like particles
p = E/c = hf/c = h/λ • Which color of light has the greatest momentum? Violet Highest f, Smallest λ
example • Calculate the momentum of an x-ray photon whose wavelength is 1.0 x 10 -10 meter Solution p = h/λ= (6.63 x 10 -34 Js) / (1.0 x 10 -10 m) = 6.6 x 10 -24 kg m/s
Matter Waves • In 1924, French Physicist, Louis De Broglie, stated that nature was symmetrical and that p = mv = h/λ should hold for both light and matter. p = momentum of the particle λ - called DeBroglie Wavelength h - Planck's constant The waves associated with matter, matter waves, do not behave as other waves in that they do not travel in space
Matter waves • The wavelength of ordinary objects are insignificant because their masses are too large.BUT… • Electrons are small enough to have wave behavior. • 1927- Diffraction and interference patterns were observed for electrons. • A scientist passed electrons through tiny double slits and observed interference patterns
example • Calculate the wavelength of a proton whose speed is 5.0 x 10^6 m/s Solution p = mv = h/λ λ = h = ( 6.6 x 10 -34 Js) . (mv) (1.66 x 10 -27 kg)(5.0 x 10 6 m/s) = 8.0 x 10 -14 m
Summary • Light behaves like a wave in interaction with large objects and like particle with very small objects like electrons
Early models of the atom http://www.montereyinstitute.org/courses/AP%20Physics%20B%20II/course%20files/multimedia/lesson52/lessonp.html
What is an Atom? • An atom is the smallest particle of an element that retains the characteristics of the element. • An atom contains protons, neutrons, and electrons. • Protons have a positive charge. • Electrons have a negative charge. • Neutrons have no charge. (They are neutral.)
Thompson’s Model of the Atom • Thomson discovered that electrons have a low mass, and that there is such a thing as a negative charge. • He concluded that because there is a negative charge, there must be a positive charge in order for an atom to be neutral. • Thought an atom consisted of a uniform distribution of positive charge in which electrons are embedded like plums in a pudding.
Rutherford’s Model • Earnest Rutherford proposed another model of the atom. • He directed a beam of massive, positively charged particles at extremely thin gold foil. • A small number of particles were scattered throughout the foil at large angles • this concentration of mass and positive charge in the atom is located at the atom’s center (nucleus). detector particles gold foil http://www.stmary.ws/highschool/physics/home/notes/modPhysics/early_models_of_atoms.htm
He concluded that the nucleus is only 1/10,000 the diameter of the average atom. • He described the atom as being a miniature solar system; the nucleus being the center where all positive charges are contained, and the outer surroundings include the electrons. • The electrons move in orbits around the nucleus and are held in orbit by Coulomb forces of attraction between their negative charges and the positive charge of the nucleus.
Did you know ... • If the atom were the size of a football stadium, the nucleus would be the size of a.... • Marble !!!!! • That's why most alpha particles go straight through!!!
Did you know ... • A neutron star is created when a massive star runs out of fuel and collapses. This tremendous gravitational collapse squeezes all the empty space out of the atom. Electrons merge with protons and create neutrons. • The density of a neutron is so enormous that a teaspoon of a neutron star would weigh 20 billion pounds!!!
Can’t be explained using Rutherford’s Model • Orbiting electrons are accelerating charges and thus should produce electromagnetic radiation. This release of energy should cause electron’s orbit to decrease leading to the collapse of atoms. But atoms are STABLE • When heated or subjected to high potential differences, atomic gases (ex. hydrogen), produce light emission spectra, rather than continuous emissions like a rainbow. WHY?
Line emission Spectra • The diagram below represents a partial line spectrum (in the visible light region). • Note: The wavelengths are in nanometers.
Niels Bohr • Early 1900's - Danish scientist Neils Bohr discovers the strange behavior of electrons. • Tried to explain why electrons were able to maintain their positions outside the nucleus rather than spiral into the nucleus and cause the atom to collapse.
The Bohr Model • In the Bohr Model the neutrons and protons (symbolized by red and blue balls in the adjacent image) occupy a dense central region called the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun (but the orbits are not confined to a plane as is approximately true in the Solar System). • Electrons in atomsare restricted in the energies they can possess, give off and absorb.
Bohr proposed • That the hydrogen atom possesses distinct orbits • At any instant, the single electron of hydrogen can be associated with one and only one orbit. • Each orbit gives the hydrogen atom a specific amount of energy (Bohr called these energy levels) • Energy levels are identified by integers – Quantum Numbers
Definitions Atom • Stationary state: when an electron is in a particular orbit • Energy level: a specific amount of energy • Ground state: when an electron is in its lowest energy level • Excited state: an electron in any level above the ground state
Energy Levels • Excitation: any process that raises the energy level of electrons in an atom • Can be the result of absorbing the energy of colliding particles of matter • Excitation energies are different for different elements • Atoms rapidly lose the energy of their various excited states as their electrons return to the ground state • Ionization potential: the energy required to remove an electron from an atom to form an ion
Bohr’s emission calculations Example: Calculate the energy of a hydrogen atom when its electron is associated with energy level2 If n is considered infinitely large, the energy of the hydrogen atom is taken to be zero because the electron is no longer considered to be associated with the atom – ionization.
The reference table has these calculated already • ΔE = Efinal – Einitial • If ΔE is a negative number, the energy is emitted; • If it is positive, the energy is absorbed • The frequency of the photon that is emitted (or absorbed)can be calculated by … • ΔE = hf
Energy Level Diagram for a Mercury atom • Notice spacing is more complex and less regular than hydrogen. • The Bohr model cannot be applied • However, we can calculate the energy of an electron transition between states E = Ei - Ef
Spectra • Atomic Spectrum: the product of when electrons in excited atoms of an element in the gaseous state return to lower energy states • Each element has a different spectrum, and is used as the “DNA” of the element
To identify an unknown element - Electrify, Heat it up and analyze its colors. - Compare emitted colors to a database of elemental spectral lines