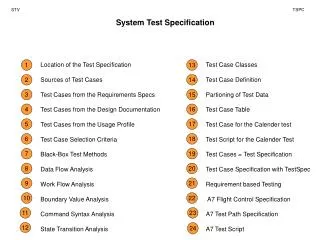
The Importance of Water: Properties and Diffusion
110 likes | 134 Vues
Explore why water is essential, including its unique properties and the process of diffusion. Discover how water forms the majority of our bodies, its polar nature, and the role of hydrogen bonds. Learn about water's resistance to temperature change, expansion when frozen, and its ability to break down rocks and form soil. Understand the concept of diffusion as the movement of molecules from crowded to less crowded areas, influenced by temperature. Discover how diffusion facilitates the transportation of food and oxygen in our organs.

The Importance of Water: Properties and Diffusion
E N D
Presentation Transcript
Why is Water Important? • Makes up 75% of us
Water cont… • Polar molecule—has a positive side and a negative side • Atoms in a covalent bond that do not share electrons equally • Ex: Oxygen is negative and hydrogen is positive in water
Water cont… • Hydrogen bond—holds two water molecules together
Water cont… • Doesn’t like change in temperature • Good insulator
Water cont… • Expands (gets bigger) when frozen • Ice is less dense (weighs less) than liquid water • Ice floats on water
Water cont… • Can break rocks apart • Will form soil over time
Diffusion • Kinetic energy—energy in motion • Anytime you move
Diffusion cont… • Moving from area that is crowded to an area with more room • Higher temperature can make diffusion happen faster
Diffusion cont… • Dynamic Equilibrium—when molecules are equal in both areas/sides
Diffusion cont… • We use diffusion to move food and oxygen into or out of our organs