Lewis Structures
170 likes | 439 Vues
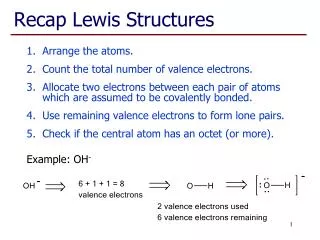
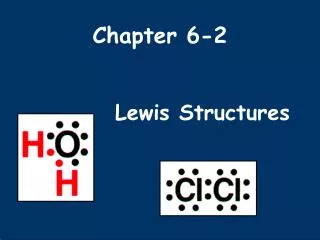
Lewis Structures. Electron-Dot-Diagrams For Molecules. Rules for Drawing Lewis Structures. Decide which atom is the central atom of the molecule Hydrogen is never a central atom (1 bond only; no lone pairs) Halogens usually make single bonds

Lewis Structures
E N D
Presentation Transcript
Lewis Structures Electron-Dot-Diagrams For Molecules
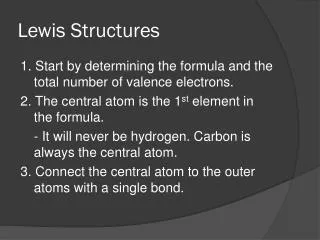
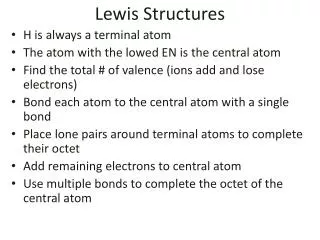
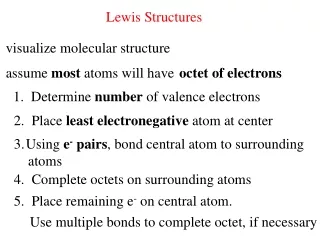
Rules for Drawing Lewis Structures • Decide which atom is the central atom of the molecule • Hydrogen is never a central atom (1 bond only; no lone pairs) • Halogens usually make single bonds • Usually the central atom is an element that there is only one of • Count all valence electrons in the molecule • Draw a line representing a covalent bond between bonded atoms • Complete the “octet” of all attached atoms
Lewis Structure Rules Cont. 5. Place remaining electrons on the central atom (in pairs) even if the octet rule is exceeded 6. If the central atom lacks an octet, form multiple bonds 7. *There are exceptions to the Octet Rule* • An excess of electrons means that the d sub shell is involved in bonding • Odd number of electrons • Not enough electrons
Vocabulary • A bond is formed when a pair of electrons is shared. (shared pairs). We indicate a bond with a line. • A double bond is formed when two pair of electrons are shared; triple when 3 pairs • A pair of electrons that is not shared is called a “lone pair”
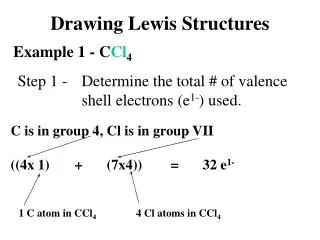
Example (PCl3) • Choose the central atom • Count valence electrons • P = 5 • Cl = 7 x 3 = 21 • Valence electrons = 26 • 3. Draw lines : : : : Cl P Cl Cl : : : : : : 4. Octet for attached atoms 5. Place remaining electrons
Example (SO3) 1. Central atom • Count valence electrons • S = 6 • O = 6 x 3 = 18 • Valence electrons = 24 • 3. Draw lines : : O S O O : 4. Octet for attached atoms : : : • No electrons remain • Form multiple bonds : : :
Example (SO42-) 1. Central atom 2. Count valence electrons S = 6 O = 6 x 4 = 24 Negative charged ion = 2 Valence electrons = 32 3. Draw lines 2- : O O S O O : : : : : 4. Octet for attached atoms : : : • Place remaining electrons • Add bracket and charge. : : :
m.socrative.comRoom #90777 • Name the following compounds: • K2SO4 • CuNO3 • SeBr2
V.S.E.P.R. Theory Valence Shell Electron Pair Repulsion
Molecular Structure Lewis structures do not necessarily illustrate molecular geometry, but they do generate the data needed to predict molecular structure. In the valence shell of the central atom of a molecule are either bonding electron pairs or nonbonding pairs of electrons. These electrons repel creating bond and lone pair angles.
VSEPR • Valence Shell Electron Pair Repulsion Theory • Shared and unshared pairs repel. • Lone pairs repel stronger than shared pairs. • Double and triple bonds are viewed as single; for structure only. • The resultant shape of the molecule is a result of shared and lone pairs being as far apart as possible.
Reactivities • The reactivity of a molecule is dependent on its shape. • The shape of a molecule is determined by its electron configuration.
Steps • Draw the Lewis structure of a compound. • Count the # bonds and lone pairs. • Apply the VSEPR rules for shape.
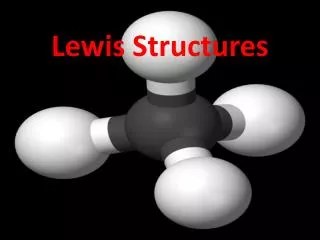
CH4 H C H H H H H-C-H H “Molecular Geometry” Complete the following table for each molecule or ion Molecule Lewis Structure Bonding Nonbonding Approx. 3-D Sketch Molecular Polar or or Ion Electron Electron Bond Shape Nonpolar Pairs Pairs Angle Molecule 0 4 109.5 tetrahedral nonpolar Multiple bonds are unidirectional and count as one area of electron density
Complete the table for each of the following molecules or ions. The central atom of each is underlined • SF2 • CF3Cl • NH3 • NH4+ • H2O • SbH3 7. CH3- 8. PF3