Understanding Lewis Structures and VSEPR Theory for Molecular Geometry
120 likes | 283 Vues
This resource provides a comprehensive guide to determining Lewis structures and applying VSEPR (Valence Shell Electron Pair Repulsion) theory to predict molecular geometry. Learn how to identify bonding and lone pairs, and ensure each atom adheres to the octet rule. Follow a step-by-step process starting with the central atom, connecting to outer atoms, and distributing valence electrons. Understand how electron repulsion influences geometric arrangements, with examples of different molecular shapes including linear, trigonal planar, and tetrahedral.

Understanding Lewis Structures and VSEPR Theory for Molecular Geometry
E N D
Presentation Transcript
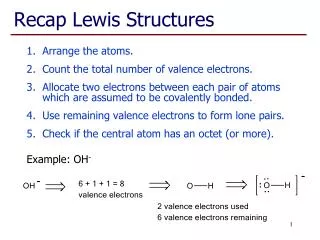
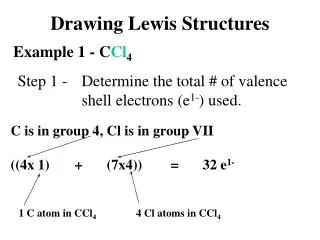
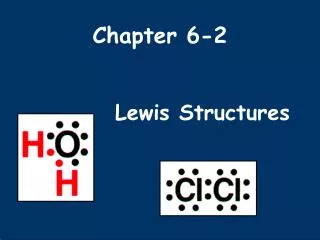
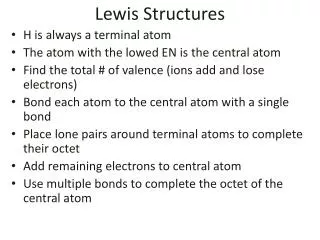
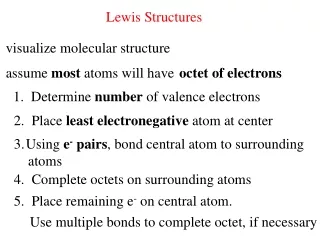
Lewis Structures 1. Start by determining the formula and the total number of valence electrons. 2. The central atom is the 1st element in the formula. - It will never be hydrogen. Carbon is always the central atom. 3. Connect the central atom to the outer atoms with a single bond.
Lewis Structures 4. Determine the number of remaining electrons. • Number from #1 – (# Bonds x 2) 5. Place the remaining electrons around the outer atoms so each obeys the octet rule. • A bond counts as 2 electrons.
Lewis Structures 6. Any remaining electrons go on the central atom. 7. Check to make sure each atom obeys the octet rule. - If the central atom does not, take a pair from an outer atom and make a double bond with the central atom.
- - - - - - Li H He VSEPR Theory • new theory: • Valence Shell Electron Pair Repulsion • “push” electron pairs as far away from each other as possible • electrons repel each other • like Thomson model of atom
look at groups of electrons • any collection of valence electrons localized around central atom • each electron group repelled by every other e- group • can be comprised of • unpaired electrons • bonding electrons • bonding • 1 group = single bond • 1 group = double bond • 1 group = triple bond • unpaired • 1 group = a single electron • e.g. free radical • 1 group = 1 lone pair
VSEPR “rules” for predicting geometry: • 1. start with valid Lewis dot structure • in case of resonance structures, any valid structure will give same geometry • 2. count number of electron groups around central atom • 3. determine number of bonding vs. unbonded electron groups • 4. describe molecular geometry • learn chart • see Table 9.2
180o 2 2 0 linear CO2 line 120o 3 3 0 trigonal planar BF3 triangle •• 120o trigonal planar bent 3 2 1 SO2 boomerang • possible geometries: total # e- groups # bond # lone pairs molecular geometry structure e.g.
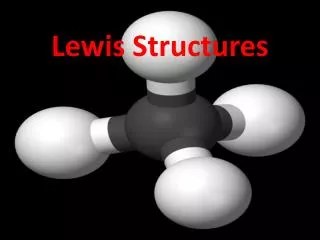
109.5o CH4 pyramid 4 4 0 tetrahedral total # e- groups # bond # lone pairs molecular geometry structure e.g.
total # e- groups # bond # lone pairs molecular geometry structure e.g. 109.5o CH4 pyramid 4 4 0 tetrahedral •• <109.5o 4 3 1 tetrahedral trigonal pyramidal NH3 tripod •• <109.5o tetrahedral bent 4 2 2 H2O “v” ••
TrigonalBipyramidal • 5 Total electron groups • 5 bonding groups • 0 lone pairs
Octahedral • 6 Total electron groups • 6 Bonded • 0 Lone Pairs